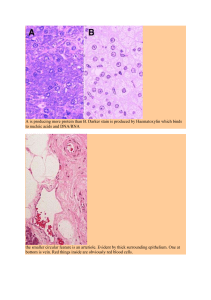
Proc. NatL Acad. Sci. USA Vol. 78, No. 9, pp. 5623-5627, September 1981 Cell Biology Isolation and characterization of human muscle cells (differentiation/contractile protein synthesis) HELEN M. BLAU AND CECELIA WEBSTER Department of Pharmacology, Stanford University School of Medicine, Stanford, California 94305 Communicated by Robert T. Schimke, May 11, 1981 Downloaded from https://www.pnas.org by 198.204.207.254 on February 6, 2023 from IP address 198.204.207.254. ABSTRACT We have developed an in vitro system for the study of postnatal human muscle under standardized conditions. The technique utilizes cloning to isolate pure populations of muscle cells. By manipulating culture conditions we can maximize either proliferation or differentiation of individual clones or of clones pooled to yield mass cultures of muscle cells. The muscle phenotype is stable; cells can be stored in liquid nitrogen for long-term use without loss of proliferative or differentiative potential. We have determined proliferative capacity of muscle cells from an analysis of clonal growth kinetics; we determined differentiative capacity from morphological evidence (cell fusion, striations, contractions, and the appearance of acetylcholine receptors) and biochemical analysis of muscle protein synthesis (creatine kinase, aactin, tropomyosin, and myosin light chains). Our approach eliminates the variability in cellular composition that has complicated studies of primary muscle to date. We can now study in a controlled fashion the interactions and contributions of different cell types to the development of normal and genetically dystrophic human muscle. Most biochemical studies of human muscle in vitro have used either explants in organ culture or dissociated monolayers of primary cells. With both techniques, muscle cells are inevitably contaminated by diverse cell types including nerve, adipocytes, and fibroblasts (see refs. 1 and 2 for reviews). It is well known that the ratio of muscle to nonmuscle cells influences the behavior ofthe muscle cells present (3) and varies in disease states (ref. 4; unpublished data). The proportion of muscle cells can be increased to 90% ofthe total cell population by preincubation on substrates not coated with collagen (5). Nonetheless, quantitative experiments using such mixed cultures remain extremely difficult. Unlike rat and mouse for which several cell lines exist (3-6), there are no established human muscle lines. As a result, previous studies of pure muscle cell populations from humans have utilized individual clones derived from single cells to analyze clonal growth kinetics and morphology (4, 7-9). Because attempts to produce differentiation of these cloned muscle cells in mass cultures have met with little success (7, 10), biochemical studies of the differentiation process have been limited. To date, the majority of studies of human muscle in tissue culture have used embryonic muscle, a source with distinct disadvantages. First, because definitive diagnosis of genetically determined muscular dystrophies is only possible postnatally, cells from fetuses have the uncertainty of, at best, a 50% risk for a disease. Second, cells isolated from the muscle of individuals of different ages have been shown to differ in the differentiative properties they express when cultured in vitro (11). Consequently, muscle from embryonic sources may not display the characteristic pathological abnormalities of the dystrophy in culture. This influence of developmental stage on cellular The publication costs ofthis article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" in accordance with 18 U. S. C. §1734 solely to indicate this fact. differentiative potential in culture is an important consideration in the analysis of normal and dystrophic myogenesis. In this report, we describe the isolation, growth, and expansion of clones of human muscle cells of postnatal origin and the conditions that maximize the proliferative or differentiative capacity of these muscle cells, either as individual clones or as pooled clones or mass cultures. The pure populations of myogenic cells can be analyzed separately or in cell mixtures of known composition in investigations ofinduction during muscle development, of cell-cell interactions at the neuromuscular junction, and of the etiology of human muscular dystrophies. MATERIALS AND METHODS Source of Human Muscle. Muscle samples were obtained from 17 normal patients during surgical treatment for orthopedic nonmuscle problems in accordance with the guidelines of the Human Subjects Committee of Stanford University. Cell Culture Conditions. Growth medium (GM) contained Ham's nutrient mixture F-10 with 0.5% chicken embryo extract and either 20% (vol/vol) fetal calf serum (GM-1) or horse serum (GM-2). Fusion medium (FM) contained Dulbecco's modified Eagle's medium and 2% (vol/vol) horse serum. Conditioned medium (CM) was GM-2 exposed to confluent human musclederived fibroblast cultures for 24 hr. filtered through a 0.2-pym Nalgene filter, and diluted 1:1 with fresh GM-2. CM can be stored at 40C for 2 weeks or at -70'C for 6 months. All cultures were grown in the presence of penicillin G (200 units/ml) and streptomycin sulfate (200 pug/ml) on a collagen substrate (calf skin collagen, Calbiochem; 1.4 mg/ml in distilled water, autoclaved). All cells were grown at 37°C in a humidified Forma incubator containing 5% CO2 and 95% air. Cells were stored frozen in 10% dimethyl sulfoxide (Mallinkrodt) in horse or fetal calf serum. F-10 and Dulbecco's modified Eagle's media, chicken embryo extract, and fetal calf serum were obtained from GIBCO, and horse serum was from Kansas City Biologicals. Horse serum lots were pretested for those that would best support fusion. Tissue culture dishes, flasks, and multiwells were from Falcon Plastics, and penicillin and streptomycin were from GIBCO. Assays for Muscle Gene Expression. Creatine kinase (CPK) activity was assayed as described (12). Synthesis of a-actin was determined by a modification of the method of Blau and Epstein (12). Cells were labeled for 3 hr in methionine-free medium supplemented with [3S]methionine (250 4Ci/ml; 1102 Ci/ mmol; Amersham) and then harvested; 0.60-2.7 X 10r cpm in 35 1Lg of protein was layered in 20 p.1 on each gel. y-, f3-, and a-actin species were resolved by fractionating labeled proteins on isoelectric focusing polyacrylamide gels, followed by autoradiography. Relative rates of actin synthesis were quantitated Abbreviations: CPK, creatine kinase; mU, milliunits; GM, growth medium; FM, fusion medium; CM, conditioned medium. 5623 Downloaded from https://www.pnas.org by 198.204.207.254 on February 6, 2023 from IP address 198.204.207.254. 5624 Cell Biology: Blau and Webster by scanning autoradiograms with a densitometer (E. C. Apparatus, St. Petersburg, FL) and cutting out and weighing the area under the relevant peaks. Other major contractile proteins were identified on two-dimensional gels (13) by comigration of labeled proteins with human contractile proteins purified directly from adult human muscle (14). The distribution of acetylcholine receptors was assayed by autoradiographic analysis of the binding of '"I-labeled-a-bungarotoxin to intact myotubes in culture (15, 16). Cells were labeled for 45 min in FM with '"I-labeled-a-bungarotoxin (specific activity, 95-140 Ci/mmol; New England Nuclear) at 50 nM, a concentration determined to be sufficient to saturate the receptors. Specificity of binding was controlled by preincubating replicate dishes of cells at each time point with unlabeled a-bungarotoxin (5 juM) for 20 min prior to labeling. For assays of total receptor, cells were solubilized in 1 M NaOH and assayed in a Micromedic 4/600 gamma counter with 75% efficiency. Total protein was determined by the method of Lowry et al. (17). Isolation and Selection of Muscle Cells from Adult Tissues. A relatively small number of cells positioned between the basement membrane and the sarcoplasmic reticulum ofadult muscle fibers are myoblasts capable of proliferation (18). After tissue dissociation, it is these satellite cells that give rise to clones in culture. Postnatal muscle samples can be stored at 40C in F-10 medium for up to 24 hr prior to dissociation without adverse effects on the yield and viability of satellite cells. For dissociation, a 0.1- to 0.3-cm3 piece of skeletal muscle tissue in F-10 medium at 40C is carefully dissected to remove as much connective tissue as possible and minced to obtain fragments smaller than 1 mm . To remove residual debris, the fragments are washed with F-10 three times at 40C and once at 370C. The tissue is then dissociated for a total of 40-60 min by two or three successive treatments with- 25 ml of 0.05% trypsin-EDTA (GIBCO) at 370C in a Wheaton graduated trypsinization flask with constant stirring. The cells collected in the supernatant after each trypsin treatment are pooled and cooled to 4°C on ice. Horse serum is added to a final concentration of 10% (vol/ vol) to terminate further protease activity. The dissociated cells are then centrifuged (2 min; 25°C); the cell pellet is resuspended in CM and either plated in culture or frozen in liquid nitrogen at a density of 0.1 cm3 of tissue per ml for future use. Enrichment of the cell population for muscle is accomplished by preplating the cells at 37C for 20 min on a non-collagencoated dish, a substrate to which fibroblasts preferentially adhere (5). Because cell counts and efficiency of plating cannot be accurately determined for human postnatal muscle cells due to debris resulting from myotube destruction during tissue dissociation, unattached cells are plated at a range of densities approximated from the expected satellite cell number, 107/cm3 of human muscle (19). Those yielding between 5 and 50 clones per 60-mm collagen-coated plate are used. Cultures are maintained in 2-3 ml of CM; they are not fed for the first 3-6 days and are fed only every 4 days thereafter. For future use, cloned cells can be frozen at a density of 5 x 106/ml. The efficacy of the isolation and selection procedure is demonstrated by the fact that 96-100% of 828 clones from four different samples were myogenic (see Table 1). RESULTS Clonal Analysis: Generation of Pure Muscle Cell Populations. A major problem in the study of postnatal human cells in culture is their limited longevity-approximately 45 doublings compared to 60-70 for muscle cells from 80-day fetuses (4). Our approach works within the time frame imposed by se- Proc. Nad Acad. Sci. USA 78 (1981) nescence. Clones containing 1000-2000 cells are harvested prior to fusion; groups of three are pooled, grown to 60-80% confluence in GM-1 in order to prevent initiation of myogenesis, and then frozen for long-term storage at a density of 5 X 106 cells per ml. Simultaneously, individual clones are tested separately for their myogenic potential by plating a few cells of each in 16-mm tissue culture wells and scoring these for fusion. This approach ensures that the frozen cells are homogeneously muscle, have not initiated differentiation, and spend a minimum of time in culture prior to use. Potential Yield of Muscle Cells per Biopsy. The yield of muscle cells from a small biopsy is sufficient for many kinds of biochemical and morphological analyses. From a 0. 1-cm3 piece of tissue, of which 50% is connective tissue and fat, 5 x 103 viable, proliferative satellite cells can be obtained. In our experience, each satellite cell is capable of giving rise to at least 1 X 107 cells, equivalent to one confluent T-75 flask or approximately 2 mg of protein. Proliferative Capacity of Frozen Stored Muscle. The proliferative capacity of frozen cells was compared with that of fresh cells. Clonal growth kinetics were determined by randomly selecting and circling clones and then visually counting the number of cells in each on subsequent days by using an inverted microscope with phase-contrast optics. Although the range in cell number per clone on day 4 suggests a high degree of heterogeneity, the growth curves are remarkably similar (Fig. 1). The apparent heterogeneity simply reflects the time in culture required for individual cells to become established and begin proliferation. For example, the clones indicated by A and 4 have lag times of 2 and 4 days, respectively, but similar growth kinetics. Table 1 shows data for the growth properties of clones of freshly dissociated and frozen-thawed cells derived from four muscle biopsy specimens. The ranges in doubling times (12-20 hr) and in the lag times prior to the initiation of proliferation (2-4 days) suggest that there is variability among clones of the same sample, of different anatomic muscles, and of different individuals with respect to these growth values. However, of importance is the observation that, on average, fresh and frozen cells behave similarly. In addition, cells frozen at the same density (0.1 cm3 of dissociated tissue per ml) but in small volumes (0.2 ml), are not adversely affected with respect to either their growth or their differentiative properties and exhibit only a slight decrease in total cell yield (data not shown). These results demonstrate that dissociated cells can be frozen in numerous aliquots for use at different times. a b 1000 ~~~~~~500- 7 / 6 6 5 - 416 0 Oi 4 ~~~50- 2 / 3 0 I~~~~~~~~1 1~ ~~~~~~~1 14] A~~~~~~~~~ nm 0 24 6 81012 1416 0 8 6 4 2 Time in culture, days Cells/clone FIG. 1. Kinetics of growth of clones of fresh cells. The number of cells in 20 individual clones was counted on day 4 (a) and daily thereafter (b); growth curves for six representatives are shown. The clones with the least and most cells are indicatd by 4 and A, respectively. Cell Biology: Blau and Webster Proc. NatL Acad. Sci. USA 78 (1981) 5625 Downloaded from https://www.pnas.org by 198.204.207.254 on February 6, 2023 from IP address 198.204.207.254. Table 1. Clonal growth and differentiation of fresh and frozen-thawed cells Muscle Aliquot Doubling Lag Fusion, sample* size, mlt time, hrt % off time, hrt Fresh: 1 20.7 ±0.8 42.2± 4.7 2 16.8 ± 0.5 89.2 ± 2.6 97 (151) 3 14.3 ± 0.6 55.0 ± 3.6 98(98) 4 13.5 ± 0.4 69.0 ± 4.4 100 (107) Mean 16.3 ± 1.6 63.9 ± 15.8 98 ± 1 Frozen: 2 1.0 14.4 ± 0.8 62.9 ± 0.8 100 (50) 2 0.2 14.9 ± 0.7 70.9 ± 3.9 96 (53) 3 1.0 12.8 ± 0.4 72.4 3.8 100 (156) 3 0.5 13.1 ± 0.4 70.8 ± 4.6 99 (121) 3 0.2 12.6 ± 0.7 61.8 ± 3.8 99 (92) Mean 13.6 ± 0.5 67.8 2.2 99 ± 1 * Each sample was from a separate muscle biopsy. Growth kinetics for individual clones of samples 3 and 4, two biopsies from the same individual, are shown in Fig. 1. Sample 2 was from the biceps femoris; all other samples were from the vastus lateralis. t Cells were frozen at the same density (0.1 cm3 of dissociated tissue per ml) but in different volumes (0.2, 0.5, 1.0 ml). t Cell doubling and lag times prior to initiation of proliferation are expressed as mean ± SEM for at least 10 clones in each case. § The percentage fusion is the proportion of total colonies that differentiated as muscle. The number of clones scored is in parentheses. Differentiative Capacity of Frozen Stored Muscle. All assays of differentiation described below were performed with mass cultures of frozen-thawed muscle cells. When plated at greater than 1 x 105 cells per 35-mm dish, frozen cells, like fresh cells, reached confluence and fused extensively until 61 ± 5% of the cell nuclei were found within myotubes. The source ofthe serum markedly affected the differentiation of these cells (Fig. 2). We have exploited this finding to grow parallel cultures of cells in low concentrations (2%, vol/vol) of horse serum to test for differentiative capacity, while maintaining the majority in a high concentration (20%, vol/vol) of fetal calf serum to promote proliferation. Decreases in serum concentration have similarly been used to promote differentiation of myogenic cells of other species (ref. 20; S. D. Hauschka, personal communication). The appearance of striations (Fig. 3) and rhythmic contractions are further evidence of differentiation. To assess differentiation biochemically, the synthesis of aactin, was compared to the synthesis of nonmuscle (& and yactins. Prior to differentiation, a-actin represented 20% of the total actin synthesized but at 7 days after a change to FM, when approximately 65% ofcells are found in myotubes, a-actin comprised 40% of the total actin synthesized (Fig. 4). We have observed similar increases in a-actin relative to total actin synthesis in primary cultures of rat and chicken [from 13% and 17% to 22% and 32%, respectively (unpublished data)], in good agreement with the findings of Garrels and Gibson (21) and Rubenstein and Spudich (22). The ratio of a- to (3 and y-actins in our differentiated cultures is not quite as high as that found in extracts of biopsied adult human muscle (data not shown). This finding is not surprising, given the persistence of unfused myoblasts in these cultures, and agrees with observations in pure populations of rat myogenic cells (21). Thus, in the course of in vitro growth and differentiation of human muscle, the synthesis of a-actin increases 2-fold and this isoform becomes the predominant actin species (Table 2). The CPK activity increased 18-fold from 140 to 2515 milliunits (mU)/mg of protein after the cells had been in FM for 5 days (Table 2). This increase was largely due to de novo synthesis of the muscle-specific isozyme (data not shown). Com- FIG. 2. Differentiation of frozen-thawed cells in mass culture. (Upper) Cells grown in medium containing horse serum differentiate. (Lower) Cells grown in medium containing fetal calf serum continue to proliferate. (x70.) parable CPK specific activities, 720 and 1000 mU/mg, have been reported in well-differentiated L6 and rat primary cells, respectively (23). Total acetylcholine receptors increased 33-fold during the course of myogenesis in vitro. This increase proved to be largely due to the production of unclustered receptors, as shown by autoradiography (Fig. 5). The rapid increase (day 3) and subsequent decrease (day 7) in receptors shown in Table 2 were observed in three independent experiments and are in good agreement with the findings of Prives et al. (24) for chicken muscle which differentiates in the absence of nerve. To stabilize receptors on the chicken myotube surface, a neural factor appears to be necessary. Peak amounts of receptor synthesized in our human cell cultures are comparable to those observed in . .-i 0-1'I. 3. Fri , .............................. .---*- .... FIG. 3. Striations in frozen-thawed cells in mass culture. Four days after growth in FM, cells were fixed in 2% glutaraldehyde in Hanks' balanced salt solution for 20 min at 37TC and stained with 2% orcein in 45% propionic acid. (x380.) 5626 B Proc. Natl. Acad. Sci. USA 78 (1981) Cell Biology: Blau and Webster iL A Downloaded from https://www.pnas.org by 198.204.207.254 on February 6, 2023 from IP address 198.204.207.254. FIG. 4. Synthesis of actins by frozen-thawed cells in mass culture. Myoblasts (A) and myotubes (B) were labeled with [55S]methionine on day 0 and day 7 after change of medium from GM-1 to FM. Proteins were separated by electrophoresis on isoelectric focusing gels and visualized by autoradiography (Upper) and densitometric scanning (Lower). muscle of other species grown in vitro (6, 24-26). The synthesis of additional muscle-specific components was determined by the fractionation of labeled proteins on two-dimensional gels (Fig. 6). Most dramatic is the initiation of synthesis of the tropomyosins and myosin light chains, similarly associated with the differentiation of muscle of other species (27-30). The isoforms observed upon differentiation of our cultures in vitro were the adult human forms; ['S]methionine-labeled proteins comigrated with accumulated myosin light chains and tropomyosins purified from adult muscle and visualized by Coomassie brilliant blue staining (data not shown). Numerous other changes in the pattern of protein synthesis occurred but have not yet been characterized. DISCUSSION Our procedure for the growth of human postnatal muscle in culture capitalizes on the presence of satellite cells, the small percentage of cells in mature muscle fibers capable of proliferation and muscle regeneration (31, 32). We have optimized conditions for growth, frozen storage, and differentiation of human cells within 45 doublings, the time frame imposed by senescence (4, 33). The total cell yield from a typical 0. 1-cm3 tissue biopsy specimen results in a minimum of 10 g of protein, an amount adequate for many kinds of biochemical and morphological analyses. FIG. 5. Distribution of acetylcholine receptors on frozen-thawed cells in mass culture. Cells were labeled with "2'-labeled-a-bungarotoxin and processed for autoradiography. (Upper) Myoblasts and myotubes as shown with phase-contrast optics. (Lbwer) The same field in dark-field illumination reveals the pattern of silver grains and the location of receptors. Arrows indicate undifferentiated myoblasts which do not have receptor. (x 100.) The proliferative and differentiative capacities of fresh and frozen muscle cells have been ascertained. The observed range in doubling times and lag times is likely to be due to minor differences in cell density and growth conditions in culture (refs. 7, 8, and 34; unpublished data). Growth kinetics, on the other hand, were more consistent among clones of a given human sample than those observed for primary or secondary rat cells (5). The ability to store small aliquots offrozen cells without loss of proliferative or differentiative capacity provides flexibility, permitting replication of experiments, easy exchange of mate- Table 2. Expression of muscle functions in mass cultures AcChoR a-Actin synthesized, % fmol/mg CPK mU/mg* protein* Days in FM total actin 0 1 2 3 5 7 0.2 0.4 140 225 795 1730 2515 2450 3.1 15.4 48.2 89.0 103.5 75.6 33 18 2 Increase, -fold Cells were plated at 1 x 105 per 35-mm dish and grown as mass cultures in GM-1 until nearly confluent. Days indicate elapsed time after change to FM. All studies were performed in duplicate; mean values are shown. * An average 35-mm dish contained 0.2 mg of protein. AcChoR, acetylcholine receptor. A *Tm ?tTm \Lc Lc\Lc ALc \; C\ L c FIG. 6. Patterns of protein synthesis in frozen-thawed cells in mass culture. Proteins synthesized by myoblasts (Left) and myotubes (Right). In each case, four pooled clones were labeled with [(5S]methionine and the proteins were assayed by two-dimensional gel electrophoresis and autoradiography. Arrows indicate muscle proteins: a-, 3-, and y-actin, tropomyosins (Tm), and four myosin light chains (Lc), identified by comigration with purified human muscle contractile proteins. Cell Biology: Blau and Webster rial among laboratories, and comparative studies of properties of normal and dystrophic muscle under identical conditions. Although the specific reasons why our conditions are effective remain unknown, it is clear that our procedure results in a degree of differentiation of mass cultures previously only observed in individual clones (7, 10). Our method differs from others in that F-10 and CM are used only to promote proliferation and not for differentiation. For optimal differentiation, we have defined a low nutrient medium with a high calcium concentration which routinely results in the formation of striated, contractile myotubes at incubator CO2 levels of 5%. Nutrient deprivation appears to enhance fusion. When F-10 is replaced by Dulbecco's modified Eagle's medium and the serum concentration is decreased from 20% to 2%, extensive differentiation occurs. In addition, the 6-fold higher calcium concentration of the latter medium compared to F-10 may be critical. pH also affects differentiation. CO2 levels >5% are inhibitory; again, the 3-fold higher bicarbonate concentration in Dulbecco's modified Eagle's medium relative to F-10 may aid in maintaining a stable pH. Under our conditions, human muscle, like muscle of other species (6, 20-29), exhibited marked increases in the synthesis of a-actin, CPK, and acetylcholine receptor. In addition, synthesis ofthe myosin light chains and tropomyosin were initiated in the course of myogenesis in vitro. It should be noted that these proteins, which are characteristic of differentiated muscle, are the adult human forms because they comigrate on gels with contractile proteins purified directly from adult human muscle tissue. -In addition, the observed increase in CPK activity is due to the de novo appearance of the muscle-specific Downloaded from https://www.pnas.org by 198.204.207.254 on February 6, 2023 from IP address 198.204.207.254. isozyme. A distinct advantage of our approach is that it uses postnatal muscle, permitting application of the methods to dystrophic muscle obtained from individuals with diagnosed genetic muscle disease rather than from fetuses at risk for a disorder. Postnatal satellite cells are also more likely to have matured sufficiently in vivo to express the relevant muscle properties in vitro (11). In summary, we have determined the requirements for a reproducible procedure for the isolation, growth, and differentiation of human muscle cell populations for in vitro study. These isolated human muscle cells will be invaluable to studies of cell-cell interactions, permitting identification of functions intrinsic to muscle and those induced or contributed by nerve, fibroblasts, or the extracellular matrix in the course of normal and genetically dystrophic human muscle development. We are grateful to Dr. D. Yaffe and Ms. B. Dieckmann for encouragement and advice early in this work, to Drs. E. Bleck and L. Rinsky for muscle samples, to Drs. S. Guttman, S. Packman, and P. Byers for critical-reading of the manuscript, and to Ms. C. Spain for expert secretarial assistance. This work was supported by grants to H. B. from the Proc. NatL Acad. Sci. USA 78 (1981) 5627 Muscular Dystrophy Association of America, National Institutes of Health Grant GM 26717, and Basil O'Connor Starter Grant 5-179 from the March of Dimes-National Foundation. 1. Rowland, L. P. (1976) Arch. Neurol. 33, 315-321. 2. Rowland, .L. P. (1980) Muscle and Nerve 3, 3-20. 3. Yaffe, D. & Saxel, 0. (1977) Nature (London) 270, 725-727. 4. Hauschka, S. D., Linkhart, T. A., Clegg, C. & Merfill, G. (1979) in Muscle Regeneration, ed. Mauro, A. (Raven, New York), pp. 311-322. 5. Richler, C. & Yaffe, D. (1970) Dev. BioL 23, 1-22. 6. Noble, M. D., Brown, T. H. & Peacock, J. H. (1978) Proc. Nati Acad. Sci. USA 75, 3488-3492. 7. Hauschka, S. D. (1974) Dev. Biol 37, 329-344. 8. Hauschka, S. D. (1974) Dev. Biot 37, 345-368. 9. Hauschka, S. D., Hainey, C., Angello, J. C., Linkhart, T. A., Bonner, P. H. & White, N. K. (1977) in Pathogenesis of Human Muscular Dystrophies, International Congress Series, ed. Rowland, L. P. (Excerpta Medica, Amsterdam), pp. 834-855. 10. Holtzer, H., Jones, K. W. & Yaffe, D. (1975)J. Neurot Sci. 26, 115-124. 11. Koenig, J. & Vigny, M. (1978) Nature (London) 271, 75-77. 12. Blau, H. M. & Epstein, C. J. (1979) Cell 17, 95-108. 13. O'Farrell, P. H. (1975) 1. BioL Chem. 250, 4007-4021. 14. Kielley, W. W. & Harrington, W. F. (1960) Biochem. Biophys. Acta 41, 401-421. 15. Blau, H. M. & Kafatos, F. C. (1978)J. Cell Biol 78, 131-151. 16. Teng, N. N. H. & Fiszman, M. Y. (1976)J. Supramot Struct. 4, 381-387. 17. Lowry, 0. H., Rosebrough, N. J., Farr, A. L. & Randall, R. J. (1951)J. Biot Chem. 193, 265-275. 18. Mauro, A. (1961)J. Biophys. Biochem. Cytot 9, 493-495. 19. Muir, A. R. (1970) in Regeneration of Striated Muscle and Myogenesis, International Congress Series, eds. Mauro, A., Shafiq, S. A. & Milhorat, A. T. (Excerpta Medica, Amsterdam), pp. 91-100. 20. Yaffe, D. & Saxel, 0. (1977) Differentiation 7, 159-166. 21. Garrels, J. I. & Gibson, W. (1976) Cell 9, 793-805. 22. Rubenstein, P. A. & Spudich, J. A. (1977) Proc. NatL Acad. Sci. USA 74, 120-123. 23. Shainberg, A., Yagil, G. & Yaffe, D. (1971) Dev. Biol 25, 1-29. 24. Prives, J., Silman, I. & Amsterdam, A. (1976) Cell 7, 543-550. 25. Betz, H. & Changeux, J.-P. (1979) Nature (London) 278, 749-752. 26. Libby, P., Bursztajn, S. & Goldberg, A. L. (1980) Cell 19, 481-491. -27. Garrels, J. I. (1979) Dev. BioL 73, 134-152. 28. Devlin, R. B. & Emerson, C. P., Jr. (1978) Cell 13, 599-611. 29. Devlin, R. B. & Emerson, C. P., Jr. (1979) Dev. BioL 69, 202-216. 30. Stockdale, F. E., Raman, N. & Baden, H. (1981) Proc. Natl Acad. Sci. USA 78, 931-935. 31. Konigsberg, U. R., Lipton, B. H. &,Konigsberg, I. R. (1975) Dev. Biol 45, 260-275. 32. Bischoff, R. (1974) Anat. Rec. 180, 645-662. 33. Hayflick, L. (1965) Exp. Cell Res. 37, 614-636. 34. Konigsberg, I. R. & Hauschka, S. D. (1965) in Reproduction: Molecular, Subcellular, and Cellular, ed. Locke, M. (Academic, New York), pp. 243-290.