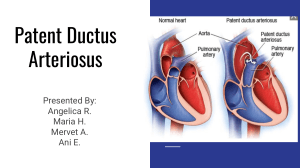
Care of at - Risk/High Risk and and Sick Mother and Child Nursing Care of the Pregnant Client Outline 4 1st Semester AY 22-23 OUTLINE A. CESAREAN BIRTH C. NURSING CARE AT Pre-op, Intra, and Post-op RISK/HIGH RISK/ SICK Management CLIENT NEW BORN 1. Problems related to maturity B. HIGH RISK Prematurity POSTPARTUM CLIENT Postmaturity 1. Post Partum Hemmorrhage 2. Problems related to Early Post Partum Gestational age Hemorrhage Small Gestational Age Late Post Partum Large Gestational Age Hemorrhage 3. Acute conditions of Hypovolemic Shock Neonates 2. Thromboembolic Disorders Respiratory Distress Superficial Venous Syndrome Thrombosis Meconium Aspiration Deep Vein Thrombosis Syndrome Pulmonary Embolism Sepsis 3. Peurperal Infection Hyperbilirubinemia Sudden Infant Death Syndrome A. CESAREAN BIRTH Although cesarean birth may be elected by some women, the procedure is used most often as a prophylactic measure to alleviate problems of birth such as cephalopelvic disproportion, breech or multiple fetus births, or failure to progress in labor. are sometimes necessary. An emergent cesarean birth carries with it the same risks of any emergent surgery: The woman may not be a prime candidate for anesthesia and may be psychologically unprepared for the experience. In addition, the woman may have a fluid and electrolyte imbalance and be both physically and emotionally exhausted from a long labor. 1. Pre-operative Management PREOPERATIVE INTERVIEW Both a woman’s primary care provider and the team member who will be administering the anesthesia interview a woman preoperatively to obtain a health history and to make assessments and decisions for safety of the procedure and the use of anesthesia. In addition to these, a nursing assessment is also essential. Be certain to ask about any past surgeries, secondary illnesses, allergies to foods or drugs, reactions to anesthesia, bleeding problems, or current medications to help establish surgical risk, and any body piercings that need to be removed because of the use of electrosurgery or an arterial cauterizing machine. In addition, include questions to discover the woman’s knowledge about: • What the procedure will entail • Length of hospitalization anticipated • If she’s been told about any postsurgical equipment to be used, such as an indwelling catheter or intravenous (IV) fluid line • Any special precautions that are being planned for her infant such as high-risk nursery care OPERATIVE RISK FOR A WOMAN Women who are in less than optimal physical or psychological health are at risk for a complicated surgical outcome unless the risk factor is identified, and special precautions are taken. Poor Nutritional Status Scheduled cesarean births are planned, which means there is time for thorough preparation for the experience throughout the antepartal period. Some women are even able to take a childbirth preparation class specifically for cesarean birth. Women who plan these need to be aware they will need epidural anesthesia, and the risk of injury to them from cesarean birth is higher than that from vaginal birth. Scheduling cesarean births this freely also can result in preterm birth with the accompanying threats to the fetus or newborn. A woman who is obese because of poor nutrition is at added risk from surgery, because tissue that contains an abundance of fatty cells is difficult to suture, thus causing the surgical incision to take longer to heal. A prolonged healing period increases the risk for infection and rupture of the incision (dehiscence). Because an obese woman’s heart has an increased workload, the physiologic shock of surgery may place greater stress on the already overworked organ. In addition, an obese woman often has more difficulty turning and ambulating postoperatively than does a woman with a lower body mass index (BMI) and therefore has an increased risk for developing respiratory or circulatory complications such as pneumonia or thrombophlebitis. A woman with a protein or vitamin deficiency is also at risk for poorer healingbecause protein and vitamins C and D are necessary for new cell formation at the incision site. Vitamin K is necessary to ensure blood clotting after surgery. Pregnant women who are iron deficient (in particular, women with a multiple gestation or women who have not taken supplements), coupled with the blood loss from surgery, are at high risk for extreme fatigue after surgery, which could interfere with parent–child bonding EMERGENT CESAREAN BIRTH Age Variations Emergent cesarean births are done for reasons that arise suddenly in labor, such as placenta previa, premature separation of the placenta, fetal distress, or failure to progress. With this second type of cesarean birth, preparation must be done rapidly but with the same concern for fully informing a woman and her support person about what circumstances created the need for the cesarean birth and how the birth will proceed. Cesarean birth is mentioned in most childbirth classes, so any woman who has taken such a class may at least understand that cesarean births Age affects surgical risk because it can cause both decreased circulatory and renal function. Fortunately, most pregnant women fall within the young adult age group, so are excellent candidates for surgery. A woman older than 40 years falls into a category of slightly higher risk not because of surgery itself but because of associated conditions such as gestational diabetes. SCHEDULED CESAREAN BIRTH Altered General Health PREOPERATIVE DIAGNOSTIC PROCEDURES A woman who has a secondary illness such as cardiac disease, diabetes mellitus, anemia, kidney, or liver disease is at greater than usual surgical risk, depending on the extent of her primary disease, because the pathology from the secondary illness may interfere with her ability to physically adjust to the demands of surgery. Therefore, asking if the woman has a secondary illness is an essential component of a preoperative nursing history. A general medication history also is important because some drugs increase surgical risk by interfering with the effect of an anesthetic or with healing of tissue. Examples of drugs that pregnant women might be taking and their potential complications are shown in Box 24.5. Preoperative assessment procedures for a woman who is to have a cesarean birth include documentation of fetal status and presentation and maturity by ultrasound assessment. In addition, assessments also include circulatory and renal function and those for all presurgery patients, including: • Vital sign determination • Urinalysis • Complete blood count • Coagulation profile (prothrombin time [PT], partial thromboplastin time [PTT]) • Serum electrolytes and pH • Blood typing and cross-matching Remember blood values need to be evaluated in light of the changes that occur with pregnancy. During pregnancy, for example, a woman (particularly one who was in prolonged labor) can have an elevated leukocyte count (up to 20,000 cells/mm3), so this finding is not as helpful an indicator for the presence of infection in the pregnant woman as it is in others. PREOPERATIVE TEACHING Women who are extremely worried about surgery need a very detailed explanation of the procedure in order to reduce their anxiety to a tolerable level. If a woman seems particularly anxious, inform the team member who will administer the anesthesia so that an antianxiety drug can be administered, if necessary, to make the experience less frightening for her. In many instances, just helping a woman acknowledge that her fear of surgery is a normal reaction can be helpful. This does not make the procedure any less traumatic, but the woman may then view her feelings as expected, which can help to enhance her self-esteem and lower anxiety. Preoperative teaching is aimed at acquainting a woman with the cesarean procedure and any special equipment to be used so she is as informed as possible. Before beginning teaching, assess how much a woman knows about her surgery. A woman who has had a cesarean birth for her first child and now is being admitted for a second procedure, for example, already knows many details. Even so, she will undoubtedly appreciate having her memory refreshed and recall confirmed. Answer all specific questions she has and fill in gaps in knowledge as necessary. Be certain all information you offer is accurate. Be certain not to use hospital jargon such as “NPO.” People under stress do not process new information well. They cannot process information at all if they do not understand the terminology. Be certain to explain the immediate preoperative measures that will be necessary, such as surgical skin preparation, eating nothing before the time of surgery, premedication (if this will be used), and method of transport to surgery. Review the necessity for an indwelling bladder catheter, IV fluid administration, and placement of an epidural catheter (if this will be used for postprocedure pain relief). For scheduled cesarean births, an explanation of not only what is going to happen immediately but also what activities should be performed to help maintain respiratory and skeletal muscle function and to prevent postsurgical complications (e.g., early ambulation) should also be included in teaching. Women who practice exercises to maintain good respiratory and circulatory function postoperatively tend to experience fewer postoperative respiratory and circulatory complications than those who do not. These preventive exercises are best taught during the preoperative period, when the woman is free of pain and can concentrate on learning. Such teaching also gives a woman a positive outlook on surgery and a sense of control over her situation. Throughout teaching, use visual aids as necessary. Draw pictures or show illustrations of anatomy, as needed. Be careful, however, not to leave textbooks about cesarean procedure techniques with a woman. Typically, these books also describe complications, and although knowledge of possible complications is necessary for informed consent, reading about complications complete with color illustrations can be overwhelming. OPERATIVE RISK TO THE NEWBORN Deep Breathing Cesarean birth places a newborn at a greater risk than does a vaginal birth. When a fetus is pushed through the birth canal, pressure on the chest helps rid the newborn’s lungs of fluid, making it easier for the baby to take a first breath. For this reason, more infants born by cesarean birth develop some degree of respiratory difficulty for a day or two after birth than those born vaginally Periodic deep breathing exercises fully aerate the lungs and help prevent stasis of lung mucus from the prolonged time spent in the supine position during surgery. Because stasis always has the potential to cause infection, preventing this helps prevent lung infection such as pneumonia. A typical exercise is to take 5 to 10 deep breaths every hour. The woman simply inhales as deeply as possible, holds her breath for a second or two, and then exhales as deeply as possible. Be certain she both inhales and exhales fully. Otherwise, she might experience light-headedness from hyperventilation. Fluid and Electrolyte Imbalance A woman who enters surgery with a lower than usual blood volume will experience the effect of surgical blood loss more than a woman who has a normal blood volume. A woman who has had a long labor before a cesarean birth is scheduled may fall into this category because she may have had little to eat or drink for almost 24 hours. Recent vomiting, diarrhea, or a chronic poor fluid intake compounds her risk. IV fluid replacement may need to be initiated preoperatively and continued postoperatively to prevent a serious fluid or electrolyte imbalance. Fear Incentive Spirometry Baseline Intake and Output Determinations A common device used three to four times a day postoperatively to encourage deep breathing is an incentive spirometer. These devices, which cause a small ping-pong–like ball to rise in a narrow tube or cause lights to flash, are both easy and fun to operate and give a woman a sense of reward for her effort. The initial impression of most people is that the device works by blowing into it. Because its purpose is to fully aerate lung spaces, however, most models are triggered by inhalation, not exhalation. A gauge can be set to monitor levels and tabs to set goals. To reduce bladder size and keep the bladder away from the surgical field, an indwelling urinary catheter may be prescribed before transport for surgery or after arrival in the surgical suite. Use good lighting so that the woman’s perineum is clearly revealed. After catheter insertion, be certain urine drains freely because fetal pressure on the urethra may considerably reduce the flow of urine. During transport, be certain to keep the drainage bag below the level of the woman’s bladder to prevent urine backflow and the possible introduction of microorganisms into the bladder. If catheterization is difficult before surgery, do not traumatize the urethra by repeated attempts as catheterization can be done in the operating room (OR) after the anesthetic agent is given. If there will be a delay between the time the catheter is inserted and the time of surgery, mark the level of drainage in the bag just before surgery or empty it, so that presurgery urine output can be differentiated from postsurgery urine output. This is because one of the gravest dangers of any surgical procedure is kidney failure from the physiologic stress of surgery or lack of blood flow to the kidneys due to decreased blood pressure. All reproductive tract surgery puts ureter flow at risk as well because the edema that collects in the surgery area can press on the ureters. Turning Be certain women understand that turning postoperatively is important to prevent both respiratory and circulatory stasis. Ambulation The most effective way to stimulate lower extremity circulation after a cesarean birth is by early ambulation. For this reason, most primary healthcare providers prefer a woman to be out of bed and walking as soon as the effect of the epidural anesthesia has worn off. Helping a woman ambulate this early can be difficult because she is both fatigued and has pain from her incision. Help her to understand ambulation is extremely important after cesarean birth because the edema from the low pelvic surgery compresses circulation to the lower extremities, thus increasing the risk for lower extremity circulatory stasis. Some women may be prescribed sequential compression devices (SCDs) or antiembolic stockings (thromboembolic devices [TEDs]) to support and encourage venous return in addition to ambulation. IMMEDIATE PREOPERATIVE CARE MEASURES A number of measures must be taken immediately before surgery to help ensure a safe outcome. Informed Consent Obtaining operative consent is the primary healthcare provider’s responsibility, but being certain, it is obtained prior to surgery is everyone’s responsibility. You may be asked to witness a woman’s signature on such a form. Before signing as a witness, be certain that it was informed consent, or one in which the risks and benefits of the procedure were explained in terms the woman could easily understand. The law differs from state to state with regard to who qualifies to be considered an emancipated or mature minor. Emancipated minors can sign their own permission for a cesarean birth, even though they are legally underage. Overall Hygiene On admission, provide a clean hospital gown. If a woman’s hair is long, encourage her to braid it or put it into a ponytail, so it will more easily fit under the surgical cap she will wear; hair contained by a cap is less likely to spread microorganisms during surgery. Follow your institution’s procedures with regard to removing nail polish, jewelry, contact lenses, lip or mouth piercings, or hair ornaments before surgery. A growing number of women wear acrylic fingernails and are reluctant to remove them for surgery. If this is the case, ensure that the woman’s toenails are free of polish so that toenails can be used to assess capillary refill if this assessment is needed. Gastrointestinal Tract Preparation A gastric emptying agent, such as metoclopramide (Reglan), to speed stomach emptying or a histamine blocker, such as ranitidine (Zantac), to decrease stomach secretions may be prescribed prior to surgery. Yet, another possibility is an oral antacid such as citric acid and sodium citrate (Bicitra), which acts to neutralize acid stomach secretions. These precautions are necessary because the woman will be lying on her back during the procedure, making esophageal reflux and aspiration highly possible. Hydration Most women have an IV fluid line begun before surgery with a fluid such as lactated Ringer’s solution. Doing so helps to ensure a woman will be fully hydrated and will not experience hypotension from epidural anesthesia administration, temporary use of a supine position, or blood loss at birth. Be certain this line is begun in the woman’s nondominant hand if possible so she can hold her newborn after surgery without interference. Use a large-size catheter or needle (18 or 20 gauge), so that blood replacement therapy can be administered by the same line if needed. Preoperative Medication A minimum of preoperative medication is used with a woman having a cesarean birth to prevent compromising the fetal blood supply and to ensure that the newborn is wide awake at birth and can initiate respirations spontaneously. Be aware if a woman has been in labor, what medications, if any, she has already received to help prevent a drug interaction. Transport to Surgery A woman may be transferred to surgery in her bed, or she may be helped to move to a stretcher. Urge her to lie on her left side during transport to prevent supine hypotension syndrome. Ensure additional safety by raising the side rails. Cover her with a blanket or sheet to avoid her feeling chilled. Check that her identification is secure before she leaves the patient unit. Make certain, even though steps are being completed rapidly, that her chart or electronic record remains secure and will be available to OR personnel. Role of the Support Person In most instances, a woman’s family can be as involved in a cesarean birth as they would be for a vaginal birth. A support person may need more encouragement to watch a cesarean than a vaginal birth, because he or she may believe the surgery will be much bloodier than it actually is. Helping family members realize cesarean birth is little different from vaginal birth not only allows them to stay with a woman during the procedure but also helps them progress to bonding with the infant and incorporating the new member into their family more easily. 2. Intraoperative Management ADMINISTRATION OF ANESTHESIA A surgical nurse will assist a woman to move from the transport stretcher or bed to the OR table and will remain with her while anesthesia is administered. If the woman has an epidural catheter in place from labor, be careful not to dislodge it while she is being moved. During transport and while in surgery, encourage the woman to remain on her side, or place a pillow under her right hip to keep her body slightly tilted to the side, to prevent supine hypotension syndrome. If a spinal anesthetic (which may be used in an emergency) is to be administered, the anesthesiologist usually will do this with the woman sitting up. The anesthesiologist may then ask you to help the woman curve her back to separate the vertebrae and facilitate entry of the spinal needle. Remember, though, that it is difficult for a woman having uterine contractions to remain in this position for long. Talking to her while letting her lean against you is the most effective means of helping her maintain this position. Epidural anesthesia is usually administered with the woman lying on her side. Duramorph is a form of morphine commonly used in addition to a local anesthesia in epidurals. Its effect lasts up to 24 hours, but because it can cause late occurring respiratory depression, respirations should be assessed every 2 hours postsurgery. SKIN PREPARATION Reducing the number of bacteria on the skin before surgery automatically reduces the possibility of bacteria entering the incision at the time of surgery. Shaving away abdominal hair, if indicated, and washing the skin area over the incision site with soap and water accomplishes this. The skin preparation area for a cesarean birth varies among agencies. Be certain to follow agency policy. To avoid being shaved, some women who are scheduled for a planned cesarean birth choose to have a bikini wax done 3 or 4 days before surgery. SURGICAL INCISION After the anesthetic administration, a woman is positioned with a towel under her right hip to move abdominal contents away from the surgical field and to lift her uterus off the vena cava. Be sure the support person is positioned at the woman’s head to provide support. Next, a screen is placed at her shoulder level and covered with a sterile drape to block the flow of bacteria from her respiratory tract to the incision site. This also helps block the woman’s and the support person’s lines of vision, thus preventing additional anxiety caused by the sight of the incision. The incision area on the woman’s abdomen is then scrubbed with an antiseptic such as iodine, and appropriate drapes are placed around the area so that only a small area of skin is left exposed. Sponge and instrument counts are simplified by the use of prepackaged cesarean birth components. Watching a cesarean birth is usually the first time a father or support person has ever witnessed surgery. Because of this, the person may be too overwhelmed by and interested in the procedure to be of optimum support. Prepare the woman and support person for the sights they might see or help talk them through them as they occur. Due in part to family and nursing staff pressure, cesarean sections have started to become more family friendly. In the past, babies were often delivered, went to the warmer for nursing assessments, and were eventually wrapped up and given to the partner to hold near the mother while surgery was finishing. More recently, several interventions have happened to make the experience more “gentle.” There are special sterile drapes that allow for a clear plastic window to be exposed, so the mother and partner can watch the baby being delivered. The opaque drape is lowered just before delivery and replaced after the newborn is delivered, so that the mother and her partner avoid watching both the initial incision and the repair. Additionally, mothers often hold their babies skin to skin after delivery, and they can attempt to breastfeed, even though the position is not ideal for latching. Cesarean sections are often not the ideal solution to delivery, but these changes can help women feel more satisfied with the experience when surgery is necessary. Types of Cesarean Incision In a classic cesarean incision, the incision is made vertically through both the abdominal skin and the uterus. The incision is made high on the uterus, so that it avoids cutting a possible placenta previa. A disadvantage of this type of incision is that it leaves a wide skin scar and also runs through the active contractile portion of the uterus. Because this type of scar could rupture during labor, if this type of incision is used, a woman will be advised not to have a subsequent vaginal birth. A low segment incision (commonly referred to as a low transverse uterine incision and a Pfannenstiel skin incision) is one made horizontally across the abdomen just over the symphysis pubis and also horizontally across the uterus just over the cervix. This is the most common type of cesarean incision used today. It is also referred to as a Misgav-Ladach or a “bikini” incision because even a low-cut bathing suit will cover the scar. Because this type of incision is through the nonactive portion of the uterus (the part that contracts minimally with labor), it is less likely to rupture in subsequent labors, making it possible for a woman to have a vaginal birth after cesarean (VBAC) in a subsequent pregnancy. VBAC rates have waxed and waned over the years along with its popularity among patients and providers, but with the recent focus on preventing cesarean births, many institutions are supportive of women who desire VBAC. The low segment incision is preferred because it: • Results in less blood loss • Is easier to suture • Decreases postpartal uterine infections • Is less likely to cause postpartum gastrointestinal complications The major disadvantage of this incision is that it takes longer to perform, possibly making it impractical for an emergent cesarean birth. In a few instances, the skin incision is made horizontally (Pfannenstiel) and then the uterine incision is made vertically or vice versa. For this reason, during a future pregnancy, do not assume a woman who has a low transverse skin incision also has had a low transverse uterine incision. BIRTH OF THE INFANT Once the surgical incision is complete, the uterus is then cut and the child’s head is born manually (Fig. 24.6). The mouth and nose of the baby may be suctioned by a bulb syringe, before the remainder of the child is born. Oxytocin (Pitocin) is administered via IV by the anesthesiologist as the child or placenta is delivered to increase uterine contraction and reduce blood loss. In many instances, a woman’s partner may be allowed to cut the umbilical cord the same as in a vaginal birth. After full birth, the uterus is pulled forward onto the abdomen and covered with moist gauze or left in the abdomen for repair. The internal cavity of the uterus is then inspected, and the membranes and placenta manually removed. If the woman wishes to have a tubal ligation or an intrauterine device (IUD) inserted for contraception, either of these can be done at this time. INTRODUCTION OF THE NEWBORN Once it is determined the newborn is breathing spontaneously, he or she is shown to the mother and support person, just as is done after a vaginal birth. Both the support person and the mother may hold the baby immediately. The mother may have some difficulty doing this. Assist her as necessary. Women are able to breastfeed after cesarean births the same as after vaginal births. However, initial breastfeeding may be delayed until the woman has been moved to a recovery room along with her infant because breastfeeding is difficult to do while still in the operating room due to position and monitors or IVs attached to the mother 3. Post-operative Management Immediately after surgery, a woman is transferred by stretcher from the OR table to the postanesthesia care unit (PACU) or a postpartal room. If spinal anesthesia was used, remember that her legs are fully anesthetized, and she will not be able to help move them. Nursing Diagnosis: Pain related to surgical incision Outcome Evaluation: Patient verbalizes extent of pain (from 1 to 10) and need for relief; states level of pain is tolerable. In the past, pain control was a major problem after cesarean birth because pain was so intense from either the uterine or abdominal incision that it interfered with a woman’s ability to move and deeply breathe. This led to surgical complications such as pneumonia or thrombophlebitis. It also made holding an infant so painful that it threatened to impair a woman’s ability to bond with her newborn. Today, a number of effective types of pain management are available, so this problem is lessened. Always use a pain rating scale to allow a woman to rate her pain. Using a specific tool helps to ensure accuracy of the assessment in light of a woman’s overall excitement at having a new child. Anxiety and fear heighten a pain response. Therefore, a woman who is concerned about her infant may rate pain higher than a woman who feels confident that her infant is doing well. In addition, a tense body posture also causes pressure on sutures. Women who had a long-action morphine epidural for labor have good pain relief up to 24 hours after birth. Others, who received a shorter acting drug for birth, need additional analgesia to be comfortable. Patient-controlled analgesia (PCA) or continued epidural injections are both effective systems for maximum pain relief. No matter what system of pain relief is used, when administering analgesics after surgery, be certain to supplement them with other comfort measures, such as urging a change of position or straightening bed linen. Always ask a woman what type of pain she is experiencing before administering a new dose of analgesia to be certain she is describing incisional or uterine pain, not pain in some other body part that would suggest a complication of surgery. Check for abdominal distention, which suggests the pain may be caused by intestinal gas rather than incision pain. If this is so, ambulation is often the most effective method to relieve this type of pain. Urge a woman to continue to take adequate analgesia to effectively manage her pain after she returns home, so she is not so distressed that she cannot nurse her infant or ambulate. Be certain she understands not to use acetylsalicylic acid (aspirin) because this can interfere with blood clotting and uterine healing. Many women who are breastfeeding are reluctant to accept any type of analgesic, especially just before breastfeeding, for fear it will pass into breast milk and to the infant. Although it is true that most analgesics do pass into breast milk, the infant takes such a small amount of breast milk (mainly colostrum) during the first days after surgery, the amount of analgesia received is negligible. Placing a pillow over her lap while the infant nurses can deflect the weight of the infant from her suture line and lessen pain. Encourage the football hold for breastfeeding as another way to keep the infant’s weight off her incision (see Chapter 19). Patient-Controlled Analgesia. With PCA, women administer doses of IV narcotic analgesia, such as morphine, to themselves by means of an IV line as needed. To receive a dose of analgesia, the woman pushes a button similar to a call bell. Thisalerts the automatic pump to deliver a set amount of narcotic into the IV line. The pump has a “lock-out” setting that prevents a woman from administering a larger dose or doses more frequently than would be safe (e.g., every 8 minutes) Patient-controlled analgesia (PCA) pump. By pushing the button, a patient delivers a bolus of narcotic to herself. With PCA, a fairly constant level of pain relief can be maintained, and pain and fear of injections are eliminated. PCA works well with postcesarean women because they feel overall well and so are interested in self-care and self-administration of analgesia. Because the narcotic is injected in such small amounts, women tend to use less analgesia with a PCA system than they would receive with intramuscular injections. Epidural Analgesia. Today, women who have epidural anesthesia for cesarean birth can have morphine (Duramorph) or fentanyl added to the epidural catheter immediately after surgery, a technique that keeps them pain free for the next 24 hours (see Chapter 16). Fentanyl creates few side effects. Although epidural morphine offers effective pain relief, side effects of administration, such as intense itching, nausea, and vomiting, can occur. An antihistamine such as diphenhydramine (Benadryl) may be needed to reduce pruritus; an antiemetic such as metoclopramide (Reglan) may be administered to counteract nausea. Even with these annoying side effects, however, epidural analgesia can be a very effective means of pain control after cesarean birth. On the postpartal unit, an infusion pump is connected to the woman’s epidural catheter, and the woman can infuse a bolus of narcotic as additional pain relief as needed. This patientcontrolled epidural analgesia (PCEA) not only is an effective means of relieving pain but also omits the problem of infiltration of an IV infusion, which can occur with IV PCA. Transcutaneous Electrical Nerve Stimulation. Transcutaneous electrical nerve stimulation (TENS) is, as the name implies, the transmission of an electrical current across the skin. Small electrodes are attached to the woman’s skin near her incision; when she feels pain, she pushes a transformer button. Irritation or stimulation of large afferent nerve fibers by the electrical stimulation blocks the ability of the smaller, paincarrying nerve fibers to transmit impulses (as predicted by gating control theory). This is the same phenomenon that rubbing or scratching skin at a point of pain achieves (see Chapter 16). The use of TENS can provide important pain relief after a cesarean birth because it gives a woman a sense of control over her situation, as does PCA or PCEA Nursing Diagnosis: Risk for deficient fluid volume related to blood loss during surgery Outcome Evaluation: Patient’s blood pressure is 100/60 mmHg; pulse remains between 60 and 100 beats/min; scant to no bleeding on surgical dressing is apparent. The potential always exists for deficient fluid volume from surgery due to blood loss until all blood vessels that were cut and ligated during surgery have thrombosed, sclerosed, and permanently sealed closed. The risk of heavy bleeding doubles for the postpartum woman because she may not only hemorrhage vaginally from a noncontracted uterus but also internally from blood vessels not yet securely closed. This danger is most acute during the first hour after surgery; it remains an acute problem for the first 24 hours. To detect the earliest signs of bleeding, monitor blood pressure, pulse, and respiratory rate approximately every 15 minutes for the first hour after surgery, every 30 minutes for the next 2 hours, every hour for the next 4 hours, or as specifically prescribed. Signs indicative of possible hemorrhage include: • Falling blood pressure (more than 20 mmHg systolic), a systolic blood pressure • less than 80 mmHg, or a drop of 5 to 10 mmHg over several readings • A change in pulse rate (greater than 110 beats/min or less than 60 beats/min) • Respirations more rapid and distressed from previous readings • Restlessness and a sense of thirst Inspect the dressing over the woman’s surgical incision for blood staining each time vital signs are assessed to document there is no incisional bleeding. Observe the perineal pad for lochia flow and palpate the fundal height each time to document uterine contraction. Lochial discharge may be decreased in a woman after a cesarean birth because the uterus was cleaned following the birth, but some lochia will always be present. It will follow a typical rubra, serosa, and alba pattern. Be certain to help a woman turn as you assess for perineal bleeding, so you can look under her body. Blood oozing vaginally or from a surgical wound can pool considerably under a woman before it is otherwise visible. Oxytocin (Pitocin) may be prescribed to be added to the first 1 or 2 L of IV fluid after surgery to ensure firm uterine contraction. If the rate of fluid administration should fall behind, be careful about “catch-up” administration. Because oxytocin (Pitocin) can elevate blood pressure by causing vasoconstriction, it may be safer to allow the fluid to remain behind for a time rather than risk elevating blood pressure by a more rapid infusion. Be aware that a woman is very prone to hemorrhage at the point the oxytocin (Pitocin) is discontinued because this is the first time her uterus is asked to maintain contraction on its own. Remember, a minimal but continued change in vital signs (e.g., pulse steadily increasing, blood pressure steadily declining) is as ominous a sign of hemorrhage, as is a sudden alteration in thesemeasurements. Notify a primary care provider of any changes in vital signs that might indicate hemorrhage so that prompt action can be taken. A woman who has had either spinal or epidural anesthesia usually will not experience pain on uterine palpation until the anesthesia has worn off in approximately 4 to 24 hours. Therefore, uterine palpation should not increase her pain. Once the effect of the anesthesia or analgesia has decreased, palpate gently enough to not cause increased pain but thoroughly enough to determine uterine consistency. At the same time you assess a woman’s uterus for firmness, assess the remainder of her abdomen for softness. A hard, “guarded” abdomen is one of the first signs of peritonitis (i.e., peritoneal infection), a complication that may occur with any abdominal surgical procedure. Nursing Diagnosis: Risk for deficient fluid volume related to postsurgical fluid restriction Outcome Evaluation: Patient’s urine specific gravity remains between 1.003 and weight loss is not more than 5 to 10 lb; fluid intake equals 2 to 3 L/day. Adequate fluid intake is important after surgery to replace blood loss from surgery and to maintain blood pressure and renal function. Because the intestine is handled during surgery, it takes approximately 24 to 48 hours before full peristaltic function is restored and oral intake is possible. It’s important IV fluids be infused during this time at a rate that is not too rapid (which could lead to cardiac overload) or too slow (which could lead to inadequate circulatory compensation). Keep an accurate intake and output record for at least the first 24 hours to be certain an adequate fluid balance has been achieved. Women are kept nothing by mouth (NPO) for a time after surgery until intestinal peristalsis has returned. To establish this is returning, assess a woman’s abdomen at least once every 8 hours for bowel sounds, such as small “pinging” sounds heard on auscultation at a rate of 5 to 10 per minute, as these demonstrate air and fluid are moving through the intestines. Passage of flatus is another indication that intestinal function is again becoming active. As soon as these signs are present, IV fluid therapy is usually discontinued and the woman is allowed sips of fluid. After she begins oral intake, wait 1 hour before removing the IV line. Waiting ensures a woman is not experiencing nausea and vomiting, which might require restarting IV therapy. Introduce oral fluid slowly (e.g., ice chips for the first hour, then sips of clear fluid such as ginger ale, Jell-O, tea, or flavored frozen ice). Gradually advance her diet to a soft and then a regular diet as prescribed. Teach women to continue to drink large quantities of fluid after they return home (at least six glasses daily), so they have adequate body fluid to make breastfeeding successful. Nursing Diagnosis: Constipation related to effects of abdominal surgery and anesthesia Outcome Evaluation: Woman voices she has a bowel movement every 2 to 3 days or her usual pattern. Carefully note the time of a woman’s first bowel movement following surgery. If she has had no bowel movement by the time of hospital discharge, her primary care provider may prescribe a stool softener, a suppository, or an enema to facilitate stool evacuation. You can reassure a woman who is not receiving much food yet that it is normal not to have bowel movements for 3 or 4 days postoperatively. Keep women’s water pitchers full to remind them to drink fluids. Urge them to eat a diet high in roughage and fluid and to attempt to move their bowels at least every other day to avoid constipation after they return home. Some women may need a stool softener prescribed to manage this because incisional pain interferes with their ability to use their abdominal muscles effectively. Caution them not to strain to pass stools because this puts pressure on their incision. Nursing Diagnosis: Risk for impaired urinary elimination related to surgical procedure Outcome Evaluation: Urinary output is more than 30 ml/hr; patient reports no pain, frequency, burning, or hesitancy on voiding. Voiding after surgery provides evidence the woman has adequate renal and circulatory function because the kidneys must have adequate blood flow through them to function. Because the bladder was handled and displaced during surgery, its tone or ability to sense filling may be inadequate to initiate voiding for the first day postsurgery. For this reason, the indwelling catheter placed before surgery is usually left in place for 4 to 24 hours to ensure good urine drainage. Assess that the catheter is draining; a postpartal woman has a urine output of 3,000 to 5,000 ml per 24 hours, so bladder distention can occur rapidly if the catheter becomes blocked. Before catheter removal, a urine culture may be requested to check for the possibility of a urinary tract infection. After removal of the catheter, the average woman will void in 4 to 8 more hours. Assess for bladder filling at the end of this time by palpation, pressing lightly over the symphysis pubis to assess fullness (Fig. 24.8A), and by percussion. On percussion, an empty bladder sounds dull; a full bladder, resonant; and an extended bladder, hyperresonant (Fig. 24.8B). If a bladder has filled to capacity but cannot empty properly or if the woman is voiding 30 to 60 ml of urine every 15 to 20 minutes, she may have retention with overflow. This voiding pattern is potentially dangerous because it means the woman’s bladder is held continuously under tension. This can result in permanent bladder damage if the condition goes undetected. In addition, the constantly full bladder may prevent the uterus from contracting, possibly increasing the risk of postpartal hemorrhage. The woman will need to be catheterized or the urinary catheter to be replaced to avoid these concerns. To help a woman void, suggest she take her prescribed analgesic to help relax abdominal musculature. In addition, assist the woman to walk to the bathroom at least every 2 hours and provide privacy. Other measures that might be effective include pouring warm water over her vulva (measure the amount of water used, so that it can be differentiated from urine) or running water from a tap within hearing distance. Teach women to continue to drink adequate fluid (at least five to six glasses daily) to ensure an adequate fluid output and to help prevent urinary tract infection after they return home. Be certain they know to telephone their primary care provider if they should develop symptoms of a urinary tract infection, such as pain with or frequency of voiding or blood in urine. Nursing Diagnosis: Risk for ineffective peripheral tissue perfusion related to immobility during and after surgery Outcome Evaluation: Capillary refill is less than 5 seconds; there is absence of calf pain, redness, edema, or areas of warmth on lower extremities. Because a woman’s abdominal muscles are lax from the stretching that occurred during pregnancy, abdominal contents tend to shift forward and put pressure on the suture line when she is sitting or standing, causing pain and an uncomfortable feelingoften described as “everything falling out.” A woman usually feels more comfortable turning and sitting up if she supports her abdomen with one hand or splints the incision with a pillow. Leg exercises such as flexing and extending her knees and early ambulation are a woman’s best safeguards against lower extremity circulatory problems. SCDs may be prescribed to help promote venous return and prevent venous stasis. Always allow a woman to sit on the edge of her bed for a few minutes before helping her to a standing position to prevent orthostatic hypotension (i.e., sudden low blood pressure that occurs with sudden position changes). Assessing that a woman’s blood pressure is adequate before she gets out of bed for the first time is an additional safeguard. Before ambulation, also assess the lower extremities for pain in the calf on dorsiflexion of the foot (i.e., Homans sign, which may or may not be reliable) or for pain, edema, warmth, or redness in the calf, to detect the possibility of a thrombus. It is dangerous for a woman to ambulate if signs of a thrombus are present. A thrombus could shift, becoming an embolus, a potentially lethal situation. Often, it is difficult for women to understand the importance of turning and ambulating as soon as possible after surgery (Fig. 24.9). Still experiencing the “taking in” postpartal phase, they may prefer to spend their first days after surgery just resting quietly in bed. Encourage women to use adequate analgesia during this time, so they can move and ambulate with the least amount of pain. Reinforce the need for continued activity balanced with rest after discharge. Be certain a woman understands the signs and symptoms of complications, such as thrombophlebitis. Nursing Diagnosis: Risk for impaired parenting related to the emergent nature of birth or discomfort from surgery Outcome Evaluation: Parents hold and feed child and voice positive comments about the infant. When a cesarean birth is unscheduled, a woman does not have much preoperative time to think about how she will feel after surgery. Most women are surprised to realize not only how well they feel overall but also how quickly they become fatigued and how painful a simple surgical incision can be. Encourage women to breastfeed, although this causes temporary uterine pain as the uterus contracts with breastfeeding. If the woman’s baby was born with a complication or has been placed in an intensive care nursery or transferred to a distant hospital for tertiary care, a woman’s postpartal course can be difficult, because she experiences a sense of loss in addition to the pain and fatigue of surgery. Depression, which can slow all body functions and certainly her ability to “take hold” in the postpartal period, can occur. Unless her baby was transferred to another site, be certain to provide her with ample time to hold and feed her child; assist her to visit in the hospital’s high-risk nursery if needed. The average woman can breastfeed satisfactorily after a cesarean birth. She may have some reason to think her baby is not quite perfect—after all, the baby was not born “perfectly”—so she may need additional time to inspect her baby and feel comfortable with him or her Nursing Diagnosis: Fatigue related to effects of surgery Outcome Evaluation: Patient states she is pleased with level of self-care; ambulates well by 24 hours, and sleeps restfully at night. Although a woman needs activity and movement after surgery, she also needs adequate rest. Many women attempt to handle their own and their newborn’s needs immediately after surgery because their excitement over their baby and their new role as a mother makes them unaware of their underlying fatigue. However, extreme fatigue interferes with healing and possibly increases the risk of infection. A woman can notice increased uterine bleeding leading to excessive loss of fluid and iron stores and, eventually, interfere with bonding. Help a woman plan a day, therefore, that includes care of her new child as well as periods of rest for herself. Be certain she has adequate analgesic medication at bedtime to allow her to be pain free for the night. Provide a space of time in the middle of the morning and again in the afternoon for uninterrupted rest while she’s still in the hospital. Explore her plans for care at home to be certain her plans for rest seem realistic for a postsurgical/postpartal woman Once she returns home, rest is often best accomplished if it is scheduled for every time her newborn sleeps. B. HIGH RISK POSTPARTUM CLIENT Late Post Partum Hemorrhage 1. Post Partum Hemmorrhage Secondary or late postpartum hemorrhage occurs 24 hours to 12 weeks postpartum. Hemorrhage, one of the primary causes of maternal mortality associated with childbearing, is a major threat during pregnancy, throughout labor, and continuing into the postpartum period. Traditionally, postpartum hemorrhage is defined as blood loss of 500 ml or more following a vaginal birth; this occurs in as many as 5% to 15% of postpartal women With a cesarean birth, hemorrhage is present when there is a 1,000-ml blood loss or a 10% decrease in the hematocrit level. Although hemorrhage may occur either early (within the first 24 hours following birth) or late (from 24 hours to 6 weeks after birth), the greatest danger is in the first 24 hours because of the grossly denuded and unprotected uterine area left after detachment of the placenta. The four main reasons for postpartum hemorrhage are uterine atony, trauma (lacerations, hematomas, uterine inversion, or uterine rupture), retained placental fragments, and the development of disseminated intravascular coagulation (DIC). These causes are generally referred to as the four T’s of postpartum hemorrhage: tone, trauma, tissue, and thrombin—a common mnemonic for the etiology of hemorrhage experienced in the puerperium. Early Post Partum Hemorrhage Early Postpartum Hemorrhage (EPH) is one of the leading causes of postpartum mortality. It is defined as blood loss of at least 500 mL after vaginal or 1000 mL following cesarean delivery within 24 hours postpartum Hypovolemic Shock Vaginal bleeding during pregnancy is always a deviation from the normal, is always potentially serious, may occur at any point during pregnancy, and is always frightening. It must always be carefully investigated because it can impair both the outcome of the pregnancy and the woman’s health or life. The primary causes of bleeding during pregnancy are summarize. Although vaginal bleeding may be innocent, any degree of this during pregnancy is a potential emergency because it may mean the placenta has loosened and cut off nourishment to the fetus. Also, the amount of blood visualized may be only a fraction of the blood actually being lost because an undilated cervix and intact membranes contain blood within the uterus. A woman with any degree of bleeding, therefore, needs to be evaluated for the possibility she is experiencing a significant blood loss or is developing hypovolemic shock. The process of shock due to blood loss is shown in Figure 21.1. Because the uterus is a nonessential body organ, danger to the fetal blood supply occurs when a woman’s body begins to decrease blood flow to peripheral organs (although the increased blood volume of pregnancy allows more than normal blood loss before hypovolemic shock processes begin). Signs of hypovolemic shock (Table 21.2) occur when 10% of blood volume, or approximately 2 units of blood, have been lost; fetal distress occurs when 25% of blood volume is lost (Box 21.2). Because “normal” blood pressure varies from woman to woman, it is important to know the baseline blood pressure for a pregnant woman when evaluating for hypovolemic shock. 2. Thromboembolic Disorders venous thrombosis that repeatedly occurs in normal veins. It may indicate a serious underlying disorder, such as cancer of an internal organ. When migratory phlebitis and cancer of an internal organ occur together, the disorder is called Trousseau syndrome. Phlebitis is inflammation of the lining of a blood vessel. Thrombophlebitis is inflammation with the formation of blood clots. Thrombophlebitis is classified as either superficial vein disease (SVD) or deep vein thrombosis (DVT). When either type occurs in the postpartum period, it tends to occur because: • A woman’s fibrinogen level is still elevated from pregnancy, leading to increased blood clotting. • Dilatation of lower extremity veins is still present as a result of pressure of the fetal head during pregnancy and birth so blood circulation is sluggish. It tends to occur most often in women who: • Are relatively inactive in labor and during the early puerperium because this increases the risk of blood clot formation • Have spent prolonged time in a birthing room with their legs positioned in stirrups • Have preexistent obesity and a pregnancy weight gain greater than the recommended weight gain, which can lead to inactivity and lack of exercise • Have preexisting varicose veins • Develop a postpartal infection • Have a history of a previous thrombophlebitis • Are older than age 35 years or have increased parity • Have a high incidence of thrombophlebitis in their family • Smoke cigarettes because nicotine causes vasoconstriction and reduces blood flow Superficial Venous Thrombosis Superficial venous thrombosis most often affects the superficial veins (veins located just under the skin) in the legs but may also affect superficial veins in the groin or in the arms. Superficial venous thrombosis in the arms usually results from having an IV. Superficial venous thrombosis in the legs usually results from varicose veins. However, most people with varicose veins do not develop blood clots (thrombosis). Even a slight injury can cause a varicose vein to become inflamed (phlebitis). Unlike deep vein thrombosis, which causes very little inflammation, superficial venous thrombosis involves a sudden (acute) inflammatory reaction that causes the blood cot (thrombus) to adhere firmly to the vein wall and lessens the likelihood that it will break loose. Unlike deep veins, superficial veins have no surrounding muscles to squeeze and dislodge a blood clot. For these reasons, superficial venous thrombosis rarely causes a blood clot to break loose (embolism). Migratory phlebitis or migratory thrombophlebitis is superficial Symptoms of Superficial Venous Thrombosis Pain and swelling develop rapidly in the area of inflammation. The skin over the vein becomes red, and the area feels warm and is very tender. Because blood in the vein is clotted, the vein feels like a hard cord under the skin, not soft like a normal or varicose vein. The vein may feel hard along its entire length. Diagnosis of Superficial Venous Thrombosis Doctors recognize superficial venous thrombosis by its appearance. Tests are not usually needed, although if people have superficial venous thrombosis above the knee that developed suddenly and not in an area of varicose veins, doctors often do ultrasonography to see if there is a blood clot in the deep veins. Treatment of Superficial Venous Thrombosis Warm compresses and analgesics for pain relief. Most often, superficial venous thrombosis subsides by itself. Applying warm compresses and taking an analgesic, such as aspirin or another nonsteroidal anti-inflammatory drug (NSAID), usually help relieve the pain. Although the inflammation generally subsides in a matter of days, several weeks may pass before the lumps and tenderness subside completely. Sometimes people who have very extensive superficial venous thrombosis are also given heparin or a different anticoagulant to help limit the blood's clotting. Deep Vein Thrombosis Deep vein thrombosis (DVT) is a medical condition that occurs when a blood clot forms in a deep vein. These clots usually develop in the lower leg, thigh, or pelvis, but they can also occur in the arm. It is important to know about DVT because it can happen to anybody and can cause serious illness, disability, and in some cases, death. The good news is that DVT is preventable and treatable if discovered early Complications of DVT The most serious complication of DVT happens when a part of the clot breaks off and travels through the bloodstream to the lungs, causing a blockage called pulmonary embolism (PE). If the clot is small, and with appropriate treatment, people can recover from PE. However, there could be some damage to the lungs. If the clot is large, it can stop blood from reaching the lungs and is fatal.In addition, one-third to one-half of people who have a DVT will have long-term complications caused by the damage the clot does to the valves in the vein called postthrombotic syndrome (PTS). People with PTS have symptoms such as swelling, pain, discoloration, and in severe cases, scaling or ulcers in the affected part of the body. In some cases, the symptoms can be so severe that a person becomes disabled.For some people, DVT and PE can become a chronic illness; about 30% of people who have had a DVT or PE are at risk for another episode. Risk Factors of DVT Almost anyone can have a DVT. However, certain factors can increase the chance of having this condition. The chance increases even more for someone who has more than one of these factors at the same time. Following is a list of factors that increase the risk of developing DVT: Injury to a vein, often caused by: o Fractures, o Severe muscle injury, or o Major surgery (particularly involving the abdomen, pelvis, hip, or legs). Slow blood flow, often caused by: o Confinement to bed (e.g., due to a medical condition or after surgery); o Limited movement (e.g., a cast on a leg to help heal an injured bone); o Sitting for a long time, especially with crossed legs; or o Paralysis. Increased estrogen, often caused by: Birth control pills Hormone replacement therapy, sometimes used after menopause Pregnancy, for up to 3 months after giving birth Certain chronic medical illnesses, such as: o Heart disease o Lung disease o Cancer and its treatment o Inflammatory bowel disease (Crohn’s disease or ulcerative colitis) Other factors that increase the risk of DVT include: o Previous DVT or PE o Family history of DVT or PE o Age (risk increases as age increases) o Obesity o A catheter located in a central vein o Inherited clotting disorders Raising and lowering your toes while keeping your heels on the floor Tightening and releasing your leg muscles Wear loose-fitting clothes. You can reduce your risk by maintaining a healthy weight, avoiding a sedentary lifestyle, and following your doctor’s recommendations based on your individual risk factors. Sympoms of DVT About half of people with DVT have no symptoms at all. The following are the most common symptoms of DVT that occur in the affected part of the body: Swelling Pain Tenderness Redness of the skin Pulmonary Embolism A pulmonary embolus is obstruction of the pulmonary artery by a blood clot; it usually occurs as a complication of thrombophlebitis when a blood clot moves from a leg vein to the pulmonary artery. The signs of pulmonary embolus are sudden, sharp chest pain; tachypnea; tachycardia; orthopnea (inability to breathe except in an upright position); and cyanosis (the blood clot is blocking both blood flow to the lungs and return to the heart). This is an emergency. A woman needs oxygen administered immediately and is at high risk for cardiopulmonary arrest. Her condition is extremely guarded until the clot can be lysed or adheres to the pulmonary artery wall and is reabsorbed. Because of the seriousness of this condition, a woman with a pulmonary embolism commonly is transferred to an intensive care unit for continuing care. 3. Peurperal Infection Infection of the reproductive tract in the postpartal period is another major cause of maternal mortality (Galvão, Braga, Gonçalves, et . Factors that predispose women to infection during this time are shown in Box 25.5. When caring for a woman who has any of these circumstances, be aware that the risk for postpartal infection is greatly increased. Prevention of DVT The following tips can help prevent DVT: Move around as soon as possible after having been confined to bed, such as after surgery, illness, or injury. If you’re at risk for DVT, talk to your doctor about: Graduated compression stockings (sometimes called “medical compression stockings”) Medication (anticoagulants) to prevent DVT. When sitting for long periods of time, such as when traveling for more than four hours: Get up and walk around every 1 to 2 hours. Exercise your legs while you’re sitting by: Raising and lowering your heels while keeping your toes on the floor Theoretically, the uterus is sterile during pregnancy and up until the membranes rupture. After rupture, pathogens can begin to invade; the risk of infection grows even greater if tissue edema and trauma are present. If infection should occur, the prognosis for complete recovery depends on such factors as the woman’s general health, virulenceof the invading organism and portal of entry, the degree of uterine involution at the time of the invasion, and the presence of lacerations in the reproductive tract. A puerperal infection is always potentially serious, because, although it usually begins as only a local infection, it has the potential to spread to the peritoneum (peritonitis) or the circulatory system (septicemia), conditions that can be fatal in a woman whose body is already stressed from childbirth. Organisms commonly cultured postpartally include group B C. NURSING CARE AT RISK/HIGH RISK/ SICK CLIENT NEW BORN streptococci, staphylococci, and aerobic gram-negative bacilli such as Escherichia coli. The management for puerperal infection focuses on the use of an appropriate antibiotic after culture and sensitivity testing of the isolated organism Box 26.3 summarizes factors associated with preterm birth. 1. Problems related to maturity Prematurity A preterm infant is traditionally defined as a live-born infant born before the end of week 37 of gestation. In terms of the degree of care needed, they are further divided into late preterm (born between 34 and 37 weeks) and early preterm (born between 24 and 34 weeks). Neonatal assessments such as inspection for sole creases, skull firmness, ear cartilage, and neurologic development plus the mother’s report of the date of her last menstrual period along with a sonographic estimation of age all can be helpful to determine gestational age. Preterm birth occurs in approximately 11% of live births worldwide, with the United States having one of the highest rates of preterm births Most preterm infants need intensive care from the moment of birth to give them their best chance of survival without neurologic aftereffects because they are more prone than others to hypoglycemia and intracranial hemorrhage. Lack of lung surfactant, because this does not form until about the 34th week of pregnancy, makes them extremely vulnerable to respiratory distress syndrome No matter what their weight, the initial assessment needs to differentiate healthy preterm babies from SGA babies (who also may have a low birth weight but have more possibility of being unhealthy and so require more help to adjust to extrauterine life). In contrast to an SGA infant, a preterm infant appears immature and has a low birth weight but is well proportioned for age because the baby appears to have been doing well in utero. For an unexplained reason, however, the trigger that initiates labor was activated too early and birth resulted even though the baby was not yet mature. Characteristics of SGA and preterm infants are compared in Table 26.1 Important among these is a high correlation between low socioeconomic level and early birth. In women from middle and upper socioeconomic groups, for example, only 4% to 8% of pregnancies are not carried to term. In women from low socioeconomic levels, as many as 10% to 20% end before term (Joseph, Fahey, Shankardass, et al., 2014). Risk factors associated with preterm birth include inadequate nutrition and smoking or alcohol use. The increasing use of assisted fertility methods that result in multiple births, such as in vitro fertilization, is another reason preterm births can occur because more multiple pregnancies result in preterm birth than term pregnancies.Iatrogenic (health-care–caused) issues, such as elective cesarean birth or inducing labor before 39 weeks of pregnancy (which is not recommended but sometimes necessary because of maternal illness or fetal reasons), also result in early births. Assessment Although a detailed pregnancy history may sometimes reveal the reason for a preterm birth, the pregnancy history is often normal up to the beginning of labor. When interviewing parents of a preterm infant, be careful not to convey disapproval of reported pregnancy behaviors such as cigarette smoking that may have contributed to preterm birth. Once an infant is born, a new mother needs a high level of self-esteem and all of her inner resources to sustain her through this crisis and not be burdened by guilt over what should or could have been. An accurate but comforting answer to a direct inquiry about why preterm birth occurs is, “No one really knows what causes prematurity.” Etiology At least 50% of neonatal deaths are preterm (. Infant mortality could be reduced dramatically if the causes of preterm birth could be discovered and corrected and all pregnancies could be brought to term. However, even with the examples of possible causes listed in the following, the exact cause of premature labor and early birth is rarely exactly known. Observing a number of physical findings and reflex testing is used to differentiate between term and preterm newborns at birth (Figs. 26.6 and 26.7). On gross inspection, a preterm infant’s head appears disproportionately large (≥3 cm greater than chest size). The skin is generally unusually ruddy because there is so little subcutaneous fat beneath it, making veins easily noticeable; a high degree of acrocyanosis may be present. Newborns delivered at greater than 28 weeks of gestation are typically covered with vernix caseosa. In very preterm newborns, however (less than 28 weeks of gestation), the vernix will be lacking. Lanugo is usually scant the same way in very low gestation infants but will be extensive, covering the back, forearms, forehead, and sides of the face in late preterm babies. Both anterior and posterior fontanelles will be small. There are few or no creases on the soles of the feet The eyes of most preterm infants appear small in relation to term infants. Although difficult to elicit, a pupillary reaction is present. An ophthalmoscopic examination is extremely difficult and often uninformative because the vitreous humor may be hazy. A preterm infant has varying degrees of myopia (nearsightedness) because of a lack of eye globe depth. The ears appear large in relation to the head. The cartilage of the ear is immature and allows the pinna to fall forward. The level of the ears should be carefully inspected to rule out chromosomal abnormalities (see Chapter 8). Neurologic function in the preterm infant is often difficult to evaluate because the neurologic system is still immature. Observing the infant make spontaneous or provoked muscle movements can be as important as formal reflex testing. If they are tested, reflexes such as sucking with coordinated swallowing and breathing will be absent if an infant’s age is below 33 weeks; deep tendon reflexes such as the Achilles tendon reflex will also be markedly diminished. During an examination, a preterm infant is much less active than a mature infant and rarely cries. If the infant does cry, the cry is weak and high pitched. 2. Problems related to Gestational age Small Gestational Age An infant is SGA (also called microsomia) if the birth weight is below the 10th percentile on an intrauterine growth curve for that age. Such infants may be born: • Preterm: before week 38 of gestation • Term: between weeks 38 and 42 • Postterm: past 42 weeks SGA infants are small for their age because they have experienced intrauterine growth restriction (IUGR) or failed to grow at the expected rate in utero. This characteristic makes them distinctly different from infants who are born with a less weight than usual but their low weight is consistent for their gestational age. Postmaturity Etiology A postterm infant is one born after the 41st week of a pregnancy . Infants who stay in utero past week 41 are at special risk because a placenta appears to function effectively for only 40 weeks. After that time, it seems to lose its ability to carry nutrients effectively to the fetus, and the fetus begins to lose weight (postterm syndrome). Infants with this syndrome demonstrate many of the characteristics of the SGA infant: dry, cracked, almost leatherlike skin from lack of fluid, and an absence of vernix. They may be SGA, and the amount of amniotic fluid surrounding them may be less at birth than usual and it may be meconium stained. Fingernails will have grown well beyond the end of the fingertips. Because they are older than a term infant, they may demonstrate an alertness much more like a 2-week-old baby than a newborn. A woman’s nutrition during pregnancy plays a major role in fetal growth, so a lack of adequate nutrition may be a major contributor to IUGR. Adolescents are prone to having a high incidence of SGA infants because if they eat only enough to meet their own nutritional and growth needs, the needs of a growing fetus can be compromised. In still other instances, the placental supply of nutrients is adequate but an infant cannot use them because of a chromosomal abnormality or an intrauterine infection such as rubella or toxoplasmosis. Even in light of these nutritional influences, the most common cause of IUGR is a placental issue: either the placenta did not obtain sufficient nutrients from the uterine arteries or it was inefficient at transporting nutrients to the fetus. Placental underdevelopment or damage, such as partial placental separation with bleeding is an example of a situation that would limit placental function because the area of placenta that separated infarcted and fibrosed, reducing the placental surface available for nutrient exchange. Women with systemic diseases that decrease blood flow to the placenta, such as severe diabetes mellitus or gestational hypertension (diseases in which blood vessel lumens are narrowed), are at higher risk for birthing SGA babies than others. Women who smoke heavily or use opiates also tend to have SGA infants When a pregnancy becomes postterm, a sonogram is usually obtained to measure the biparietal diameter of the fetus. A nonstress test or complete biophysical profile may be done to establish whether the placenta is still functioning adequately. A cesarean birth may be indicated if a nonstress test reveals that compromised placental functioning is apt to occur during labor. At birth, the postterm baby is likely to have difficulty establishing respirations, especially if meconium aspiration occurred. Polycythemia may have developed from decreased oxygenation in the final weeks. The hematocrit may be elevated because polycythemia and dehydration have lowered the circulating plasma level. In the first hours of life, hypoglycemia may develop because the fetus had to use stores of glycogen for nourishment in the last weeks of intrauterine life. Subcutaneous fat levels may also be low, having been used in utero. This loss of fat can make temperature regulation difficult, making it important to prevent a postterm infant from becoming chilled at birth or during transport. Any woman is anxious when she does not have her baby on her due date. She is apt to become extremely anxious and perhaps angry when it is determined her baby is postterm. It seems that, if her baby stayed so long in utero under her protection, the baby should be extra healthy and strong. Why, then, she asks, is her baby being transferred for special care? The mother may also feel guilty for not providing well for her infant in the last few weeks of the pregnancy. Make certain a woman spends enough time with her newborn to assure herself that although birth did not occur at the predicted time, the baby should do well with appropriate interventions to control possible hypoglycemia or meconium aspiration. All postterm infants need follow-up care until at least school age to track their developmental abilities because the lack of nutrients and oxygen in utero may have left them with neurologic symptoms that will not become apparent until they attempt finemotor tasks Assessment The SGA infant may be detected in utero when fundal height during pregnancy becomes progressively less than expected. However, if a woman is unsure of the date of her last menstrual period, this discrepancy can be hard to substantiate; a sonogram can then demonstrate the decreased size. A biophysical profile including a nonstress test, placental grading, amniotic fluid amount, and an ultrasound examination documents additional information on placental function and fetal growth. If poor placental function is apparent from such determinations, it can be predicted that the infant will do poorly during labor during the periods of relative hypoxia, which occur during contractions. Cesarean birth, therefore, is the birth method of choice in such circumstances. Appearance Generally, an infant who suffers nutritional deprivation early in pregnancy, when fetal growth consists primarily of an increase in the number of body cells, is below average in weight, length, and head circumference. An infant who suffers deprivation late in pregnancy, when growth consists primarily of an increase in cell size, may have only a reduction in weight. Regardless of when deprivation occurs, the infant tends to have an overall wasted appearance. The infant may have poor skin turgor and generally appears to have a large head because the rest of the body is so small. Skull sutures may be widely separated. Hair may be dull and lusterless. The infant may have a small liver, which can cause difficulty regulating glucose, protein, and bilirubin levels after birth. The abdomen may be sunken. The umbilical cord often appears dry and may be stained yellow. In contrast, because an infant’s age is more advanced than the weight implies, an infant may have better developed neurologic responses, sole creases, and ear cartilage than expected for a baby of that weight. The infant may also seem unusually alert and active. As a first assessment, the SGA infant needs to be examined carefully for possible congenital anomalies that occurred because of the poor nutritional intrauterine environment. Laboratory Findings Blood studies at birth usually show a high hematocrit level (less than normal amounts of plasma in proportion to red blood cells are present because of a lack of fluid) and an increase in the total number of red blood cells (polycythemia). The increase in red blood cells occurs because anoxia during intrauterine life stimulated excess development of them. An immediate effect of polycythemia is to cause increased blood viscosity, a condition that puts extra work on the infant’s heart because it is more difficult to effectively circulate thick blood. As a consequence, acrocyanosis (blueness of the hands and feet) may be prolonged and persistently more marked than usual. If the polycythemia is extreme, vessels may actually become blocked and thrombus formation can result. If the hematocrit level is more than 65% to 70%, an exchange transfusion to dilute the blood may be necessary. A second problem of polycythemia is hyperbilirubinemia because so many extra red blood cells break down and release bilirubin. Because SGA infants have decreased glycogen stores, still another common problem that develops is hypoglycemia (decreased blood glucose, or a level below 45 mg/dl). Such infants may need intravenous glucose to sustain blood sugar until they are able to suck vigorously enough to take sufficient oral feedings. Large Gestational Age An infant is LGA (also termed macrosomia) if the birth weight is above the 90th percentile on an intrauterine growth chart for that gestational age. Such a baby appears deceptively healthy at birth because of the weight, but a gestational age examination often reveals immature development. It is important that LGA infants be identified immediately so they can be given care appropriate to their gestational age rather than being treated as term newborns Etiology Infants who are LGA have been subjected to an overproduction of nutrients and growth hormone in utero. This happens most often to infants of women who are obese or who have diabetes mellitus. Multiparous women may also have large babiesbecause with each succeeding pregnancy, babies tend to grow larger. Beckwith–Wiedemann syndrome, a rare condition characterized by general body overgrowth and congenital anomalies such as omphalocele, may also be a cause. Assessment A fetus is suspected of being LGA when a woman’s uterus appears to be unusually large for the date of pregnancy. Abdominal size can be deceptive, however. Because a fetus lies in a flexed fetal position, he or she does not occupy significantly more space at 10 lb than at 7 lb. If a fetus does seem to be growing at an abnormally rapid rate, a sonogram can confirm the suspicion. A nonstress test to assess the placenta’s ability to sustain a large fetus during labor may be prescribed. Lung maturity may be assessed by amniocentesis. If an infant’s large size was not detected during pregnancy, it may be first recognized during labor when the baby appears too large to descend through the pelvic rim. If this happens, a cesarean birth may be necessary because shoulder dystocia (the wide fetal shoulderscannot pass; or needs significant manipulation to pass through the outlet of the pelvis) would halt vaginal birth at that point. Appearance At birth, LGA infants may show immature reflexes and low scores on gestational age examinations in relation to their size. They may have extensive bruising or a birth injury such as a broken clavicle or Erb–Duchenne paralysis from trauma to the cervical nerves if they were stressed in order for the wide shoulders to be born vaginally (see Chapter 51). Because the head is large, it may have been exposed to more than the usual amount of pressure during birth, causing a prominent caput succedaneum, cephalohematoma, or molding. Because LGA newborn are large but often immature, they require the same cautious care necessary for a preterm infant. Cardiovascular Dysfunction Polycythemia may occur in an LGA fetus as the fetus attempts to fully oxygenate more than the average amount of body tissue. Following birth, observe LGA infants closelfor signs of hyperbilirubinemia that may result from absorption of blood from bruising and breakdown of the extra red blood cells created by polycythemia. Assess the infant’s heart rate also. If cyanosis is present, it may be a sign of poor heart function, but it could also be from transposition of the great vessels, a serious heart anomaly associated with macrosomia. Hypoglycemia LGA infants also need to be carefully assessed for hypoglycemia in the early hours of life because large infants require large amounts of nutritional stores to sustain their weight. If the mother had diabetes that was poorly controlled (the cause of the large size), the infant would have had an increased blood glucose level in utero to match the mother’s glucose level; this caused the infant to produce elevated levels of insulin. After birth, these increased insulin levels will continue for up to 24 hours of life, possibly causing rebound hypoglycemia. 3. Acute conditions of Neonates Respiratory Distress Syndrome Respiratory distress syndrome (RDS) of the newborn, formerly termed hyaline membrane disease, is most often seen in newborns born prematurely. Other causes of RDS include newborns with meconium aspiration syndrome, sepsis, a newborn who is slow to transition to extrauterine life, and pneumonia. The pathologic feature of RDS is a hyalinelike (fibrous) membrane formed from an exudate of an infant’s blood that begins to line the terminal bronchioles, alveolar ducts, and alveoli. This membrane prevents the exchange of oxygen and carbon dioxide at the alveolar– capillary membrane, interfering with effective oxygenation. The cause of RDS is a low level or absence of surfactant, the phospholipid that normally lines the alveoli and reduces surface tension to keep the alveoli from collapsing on expiration. Because surfactant does not form until the 34th week of gestation, as many as 30% of LBW infants and as many as 50% of VLBW premature infants are susceptible to this complication. Pathophysiology High pressure is required to fill the lungs with air for the first time and overcome the pressure of lung fluid. For example, it takes a pressure between 40 and 70 cm H2O to inspire a first breath but only 15 to 20 cm H2O to maintain quiet, continued breathing. If alveoli collapse with each expiration, as happens when surfactant is deficient, forceful inspirations requiring optimum pressure are still required to inflate them. Even very immature infants release a bolus of surfactant at birth into their lungs from the stress of birth. However, with deficient surfactant, areas of hypoinflation begin to occur and pulmonary resistance increases. Blood then shunts through the foramen ovale and the ductus arteriosus as it did during fetal life. The lungs become poorly perfused. As a result, the production of surfactant decreases even further. The poor oxygen exchange that results leads to tissue hypoxia, which causes the release of lactic acid. This, combined with the increasing carbon dioxide level resulting from the formation of the hyaline membrane on the alveolar surface, leads to severe acidosis. Acidosis causes vasoconstriction and decreased pulmonary perfusion from vasoconstriction, which further limits surfactant production. With surfactant production almost lost, the ability to stop alveoli from collapsing with each expiration becomes more and more difficult. This vicious cycle continues until the oxygen–carbon dioxide exchange in the alveoli is no longer adequate to sustain life without ventilator support. Assessment Most infants who develop RDS have difficulty initiating respirations at birth. After resuscitation, they appear to have a period of hours or a day when they are free of symptoms because of an initial release of surfactant. During this time, however, subtle signs may appear, such as: • Low body temperature • Nasal flaring • Sternal and subcostal retractions • Tachypnea (more than 60 breaths/min) • Cyanotic mucous membranes Within several hours, expiratory grunting occurs caused by closure of the glottis as it tries to increase the pressure in alveoli on expiration in order to help to keep them from collapsing. Even with this attempt at better oxygen exchange, however, as the disease progresses, infants become cyanotic and their Po2 and oxygen saturation levels fall in room air. On auscultation, there may be fine rales and diminished breath sounds because of poor air entry. As distress increases, an infant may exhibit: • Seesaw respirations (on inspiration, the anterior chest wall retracts and the abdomen protrudes; on expiration, the sternum rises) • Heart failure, evidenced by decreased urine output and edema of the extremities • Pale gray skin • Periods of apnea • Bradycardia • Pneumothorax It’s important the infant is tipped to an upright position following administration and the infant’s airway is not suctioned for as long as safely possible after administration of surfactant to help it reach lower lung areas and to avoid suctioning the drug away. Although there are almost no unfavorable reactions to surfactant administration, some, such as mucus plugging from the solution, do occur. An infant who is receiving surfactant and then is placed on a ventilator needs close observation because lung expansion can improve so rapidly, the ventilator pressure becomes too high. Anticipate the need to adjust ventilator settings to accommodate the vastly improved lung function. Oxygen Administration The administration of oxygen is often necessary to maintain correct Po2 and pH levels following surfactant administration, and it may be administered in a variety of ways from a simple cannula or mask, continuous positive airway pressure (CPAP), or assisted ventilation with positive end-expiratory pressure (PEEP). The advantage of CPAP or PEEP is that this exerts pressure on the alveoli at the end of expiration and helps keep alveoli from collapsing in addition to supplying oxygen. Highfrequency, oscillatory, and jet ventilation are still other methods of introducing oxygen to infants with noncompliant lungs. These systems maintain airway pressure and then intermittently “jet” or oscillate an additional amount of air at a rapid rate (400 to 600 times per minute) to inflate alveoli. A possible complication of oxygen therapy in the very immature or very ill infant is ROP (see discussion later in chapter) or bronchopulmonary dysplasia (BPD) which is also known as chronic lung disease (see Chapter 40). Ventilation The diagnosis of RDS is made on the clinical signs of grunting, central cyanosis in room air, tachypnea, nasal flaring, and retractions. A chest X-ray will reveal a diffuse pattern of radiopaque areas that look like ground glass (haziness) in the lungs. Blood gas studies will reveal respiratory acidosis. A βhemolytic, group B streptococcal infection may mimic RDS because this infection is so severe in newborns that it stops surfactant production. Cultures of blood, cerebrospinal fluid, and skin may be obtained, therefore, to rule out this condition. An antibiotic (penicillin or ampicillin) and an aminoglycoside (gentamicin or kanamycin) may be started while culture reports are pending. Normally, on a ventilator, inspiration is shorter than expiration, or there is an inspiratory/expiratory (I/E) ratio of 1:2. It is difficult to deliver enough oxygen to stiff, noncompliant lungs in this usual ratio, however, without forcing the air into the lungs at such a high pressure and rapid rate that a pneumothorax becomes a constant concern. Infant ventilators are therefore available with a reversed I/E ratio (2:1). These are pressure cycled to control the force with which air is delivered. Complications of any type of ventilation are possible, such as pneumothorax and impaired cardiac output because of decreased blood flow through the pulmonary artery from increased lung pressure. There is also a possible risk of increased intracranial and arterial pressure and hemorrhage from fluctuating blood pressures. Being certain infants are not overhydrated is important to help prevent increased blood pressure and increased pulmonary artery pressure, which may delay the closure of the ductus arteriosus and interfere with both heart and lung function. Therapeutic Management Additional Therapy: Nitric Oxide Surfactant Replacement RDS can be largely prevented by the administration of surfactant at birth for an infant at risk because of low gestational age (Box 26.7). Immediately after birth, synthetic surfactant is administered into an endotracheal tube by a syringe or catheter (lung lavage). An additional therapy that can help to oxygenate a newborn’s lungs is the administration of nitric oxide, a potent vascular dilator. It causes pulmonary vasodilation without decreasing systemic vascular tone. It combines with hemoglobin in the intravascular space to form methemoglobin. This causes systemic vasodilation. The nitric oxide enters the alveoli on ventilation and redirects the pulmonary blood by dilating the pulmonary arterioles. Extracorporeal Membrane Oxygenation Extracorporeal membrane oxygenation (ECMO) was first developed as a means of oxygenating blood during cardiac surgery. Its current use has expanded to include the management of severe hypoxemia in newborns with illnesses such as meconium aspiration, RDS, pneumonia, and diaphragmatic hernia. Formerly used as a mainstay of therapy for RDS, it is now rarely needed because surfactant lavage is so effective. Supportive Care An infant with RDS must be kept warm because cooling increases acidosis in newborns, and for the newborn with RDS, acidosis may increase to lethal levels. Keeping an infant warm also reduces the infant’s metabolic oxygen demand. Provide hydration and nutrition with intravenous fluids and glucose or gavage feedings because the respiratory effort makes an infant too exhausted to suck. Prevention RDS rarely occurs in mature infants. Dating a pregnancy by sonogram and by documenting if the level of lecithin in surfactant obtained from amniotic fluid exceeds that of sphingomyelin by a 2:1 ratio are both important ways to be certain an infant born by cesarean birth or for whom labor is induced is mature enough that RDS is not likely to occur. Using a tocolytic agent such as magnesium sulfate can help prevent preterm birth for a few days. During this time, if a woman receives two injections of a glucocorticosteroid, such as betamethasone, it may be possible to prevent RDS in the newborn because steroids appear to quicken the formation of lecithin. The administration is most effective when given between weeks 24 and 34 of pregnancy. Unfortunately, there is often no warning that preterm birth is imminent until hours before birth. Because the steroid does not take effect before 24 to 48 hours, some labors and births will progress too rapidly for this preventive measure to be effective. Meconium Aspiration Syndrome Meconium is present in the fetal bowel as early as 10 weeks of gestation. If hypoxia occurs, a vagus reflex is stimulated, resulting in relaxation of the rectal sphincter. This releases meconium into the amniotic fluid. Babies born breech may expel meconium into the amniotic fluid from pressure on the buttocks. In both instances, the appearance of the fluid at birth is green to greenish black from the staining. Meconium staining occurs in approximately 10% to 20% of all births; in 2% to 4% of these births, infants will aspirate enough meconium to cause meconium aspiration syndrome (MAS). Meconium aspiration does not tend to occur in ELBW infants because the substance has not passed far enough in the bowel for it to be at the rectum in these infants. An infant may aspirate meconium either in utero or with the first breath at birth. Meconium can cause severe respiratory distress (tachypnea, retractions, and grunting). The infant may also require increased oxygen to maintain saturations in the mid to upper 90s. This oxygen requirement usually starts in the first couple hours after birth without any congenital anomalies that may cause the low oxygen saturations. Assessment Infants with meconium-stained amniotic fluid can have difficulty establishing respirations at birth (those who were not born breech have had a hypoxic episode in utero to cause the meconium to be in the amniotic fluid). The Apgar score is apt to be low. Almost immediately, tachypnea, retractions, and cyanosis begin. The infant should be placed on the warmer, and resuscitation should begin including the initiation of positive pressure ventilation as necessary. After the initiation of respirations, an infant’s respiratory rate may remain rapid (tachypnea) and coarse bronchial sounds may be heard on auscultation. The infant may continue to have retractions because the inflammation of bronchi tends to trap air in the alveoli, limiting the entrance of oxygen. This air trapping may also cause enlargement of the anteroposterior diameter of the chest (barrel chest). Pulse oximetry or blood gases will reveal poor gas exchange evidenced by a decreased PO2 and an increased PCO2. A chest X-ray will show bilateral coarse infiltrates in the lungs, with spaces of hyperaeration (a peculiar honeycomb effect). The diaphragm will be pushed downward by the overexpanded lungs. Therapeutic Management Amnioinfusion can be used to dilute the amount of meconium in the amniotic fluid and has shown to improve the outcomes for the newborn with meconium in situations where perinatal observation is limited. The benefits may be related to dilution of the meconium or having an effect on the oligohydramnios (Hofmeyr, Xu, & Eke, 2014). If deeply stained amniotic fluid is identified during labor, the infant may be scheduled for a cesarean birth. After birth, infants may need to be treated with oxygen administration and assisted ventilation. Antibiotic therapy may be prescribed to forestall the development of pneumonia as a secondary problem. If lung compliance is poor, surfactant may be administered. If lung noncompliance continues, this may necessitate high inspiratory pressure. Unfortunately, this can cause a pneumothorax or pneumomediastinum (air in the chest cavity). Observe the infant closely, therefore, for signs of trapping air in the alveoli because the alveoli can expand only so far and then will rupture, sending air into the pleural space (pneumothorax). Yet, a further complication that can occur because of increased pulmonary resistance is the ductus arteriosus remaining open, causing blood to shunt from the pulmonary artery into the aorta and compromising cardiac efficiency and increasing hypoxia. To detect this, observe an infant closely for signs of heart failure such as increased heart rate or respiratory distress. Maintain a temperature-neutral environment to prevent the infant from having to increase metabolic oxygen demands. A chest physiotherapy with percussion and vibration may be helpful to encourage the removal of remnants of meconium from the lungs (see Chapter 40). Some infants may need to be administered nitric oxide or maintained on ECMO to ensure adequate oxygenation Sepsis Newborns are susceptible to infections during pregnancy and at birth because their ability to produce antibodies is immature. A number of infections in newborns, such as toxoplasmosis, rubella, syphilis, and cytomegalovirus infections, spread to the fetus across the placenta in utero and are discussed in Chapter 12 with other complications of pregnancy. Other infections, such as those discussed in the following sections, are not contracted in utero but are contracted from exposure to vaginal secretions at birth. β-HEMOLYTIC, GROUP B STREPTOCOCCAL INFECTION A serious cause of infection in newborns is the gram-positive βhemolytic, group B streptococcal (GBS) organism, a natural inhabitant of the female genital tract. Between 50 and 300 infants out of every 1,000 live births display a positive culture for the organism (AAP, 2011a). It also may be spread from baby to baby if good hand washing technique is not used in caring for newborns. If a woman is found to be positive for GBS during late pregnancy (see Chapter 21), ampicillin administered IV during pregnancy and again during labor helps to reduce the possibility of newborn exposure. Assessment Universal screening is recommended for pregnant women at 35 to 37 weeks of gestation to see if they have GBS organisms in their vaginal secretions. Typically, a newborn at risk, such as one born after prolonged rupture of membranes or if the woman’s vaginal culture is positive for GBS, will be screened at birth for infection by a specialized GBS blood culture. Colonization by GBS can result in either an early-onset or a lateonset illness. With the early-onset form, signs of pneumonia such as tachypnea, apnea, extreme paleness, hypotension, or hypotonia become apparent within the first day of life. Decreased urine output can occur from the hypotension. A chest X-ray may not be diagnostic because the changes seen are almost indistinguishable from those of RDS (a ground-glass appearance). Without therapy, the disease progresses so rapidly, as many as 20% of infants who contract the infection die within 24 hours of birth. A late-onset type occurs at 2 to 4 weeks of age. With this, instead of pneumonia being the infection focus, meningitis tends to occur. Typical signs include lethargy, fever, loss of appetite, and bulging fontanelles from increased intracranial pressure. Mortality from the late-onset type is not as high as that from the early-onset form (15% vs. 20%), but neurologic consequences can occur in up to 50% of infants who survive. Therapeutic Management If a newborn displays signs of infection or a blood screening test is positive, antibiotics such as penicillin, cefazolin, clindamycin, or vancomycin are all effective against the GBS organism. Parents may have difficulty understanding how their infant could suddenly have become this ill, and they may need a great deal of support to care for their infant. This is even more important if the newborn survives the infection but is left neurologically challenged. In the future, immunization of all women of childbearing age against streptococcal B organisms could decrease the incidence of newborns infected at birth. OPHTHALMIA NEONATORUM Ophthalmia neonatorum is an eye infection that occurs at birth or during the first month of life. The most common causative organisms are Neisseria gonorrhoeae and Chlamydia trachomatis, which are contracted from vaginal secretions. An N. gonorrhoeae infection is an extremely serious form of infection because, if left untreated, the infection progresses to corneal ulceration and destruction, resulting in opacity of the cornea and severe vision impairment. Assessment Ophthalmia neonatorum is generally bilateral. The conjunctivae become fiery red and covered with thick pus. The eyelids appear edematous. Although this usually occurs on day 1 to day 4 of life, it should be considered as a possibility when conjunctivitis occurs in any infant younger than 30 days of age. Prevention The prophylactic instillation of erythromycin ointment into the eyes of newborns prevents both gonococcal and chlamydial conjunctivitis. In the past, eye prophylaxis was given immediately after birth so it was never forgotten. Now it is more customary to delay the administration of the ointment until after the first reactivity period so the newborn can clearly see the parents during this important attachment period. This makes it easy for administration to be forgotten, so use some type of a checklist as a reminder of this important prophylaxis. Infants born outside the hospital also need prophylaxis to prevent ophthalmia neonatorum, the same as for infants born in a birthing room. Therapeutic Management If conjunctivitis occurs, therapy is individualized depending on the organism cultured from the exudate. If gonococci are identified, intravenous ceftriaxone (Rocephin) and penicillin are effective drugs. If Chlamydia is identified, an ophthalmic solution of erythromycin is commonly used. Use standard and contact infection precautions when caring for this newborn. In addition to systemic antibiotic therapy, sterile saline solution lavage to clear the copious discharge from the eyes may be prescribed. When irrigating eyes, use a sterile medicine dropper or bulb syringe and use barrier protection, including goggles to avoid splashing any solution into your own eye. The solution should be at room temperature. Direct the stream of the irrigation fluid laterally so it does not enter and contaminate the other eye. The mother of the infected infant needs treatment for gonorrhea or chlamydia before fallopian tube sterility or pelvic inflammatory disease can result. Sexual contacts of the mother should be treated also so the spread of the disease can be halted. With either infection, parents can be assured with early diagnosis and treatment that the prognosis for normal eyesight in their child is good. HEPATITIS B VIRUS INFECTION Hepatitis B virus (HBV) can be transmitted to the newborn through contact with infected vaginal blood at birth when the mother is positive for the virus (positive for the surface antigen of the hepatitis B virus [HBsAg+]). Hepatitis B is a destructive illness with greater than 90% of infected infants becoming chronic carriers of the virus as well as the risk of developing liver cancer later in life (Ni, 2011). To reduce the possibility of HBsAg being spread to newborns in the future, parents are asked if they would like their infant vaccinated against hepatitis B at birth (Kurosky, Davis, & Krishnarajah, 2016). If the mother is identified as HBsAg+, her infant should be bathed as soon as possible after birth to remove HBV-infected blood and secretions. Gentle suctioning is necessary to avoid trauma to the mucous membrane, which could allow HBV invasion. To further protect against infection, the infant is administered serum hepatitis B immune globulin (HBIG) in addition to the HBV vaccination. Although the virus is transmitted in breast milk, once immune globulin has been administered, women may breastfeed without risk to an infant. Hepatitis B is further discussed in Chapter 45 because it shares common symptoms with other liver disorders and also occurs in older children. GENERALIZED HERPESVIRUS INFECTION A herpes simplex virus type 2 (HSV-2) infection, which is most prevalent among women with multiple sexual partners, can be contracted by a fetus across the placenta if the mother has a primary infection during pregnancy. More often, however, the virus is contracted from the vaginal secretions of a mother who has active herpetic vulvovaginitis at the time of birth. Between 15% and 30% of women of childbearing age demonstrate antibodies to this virus or have the potential to have active lesions during labor;. Assessment If the infection was acquired during pregnancy, an infant may be born with vesicles covering the skin. The long-term prognosis of the child is guarded because severe neurologic damage may have occurred simultaneously with the development of the lesions. If infants don’t acquire the infection until birth, by day 4 to day 7 of life, they show a loss of appetite, perhaps a low-grade fever, and lethargy. Stomatitis (ulcers of the mouth) or a few vesicles on the skin appear. Herpes vesicles always cluster, are pinpoint in size, and are surrounded by a reddened base. After the vesicles appear, infants become extremely ill. They develop dyspnea, jaundice, purpura, convulsions, and hypotension. Death may occur within hours or days. Between 25% and 70% of newborns who survive generalized herpesvirus infections have permanent central nervous system sequelae. To confirm the diagnosis, cultures are obtained from representative vesicles as well as from the nose, throat, anus, and umbilical cord. Blood serum is analyzedfor IgM antibodies. Therapeutic Management An antiviral drug such as acyclovir (Zovirax), a drug that inhibits viral DNA synthesis, is effective in combating this overwhelming infection. Prevention, however, is the newborn’s best protection. Antenatal antiviral prophylaxis reduces viral shedding and recurrences at birth and reduces the need for cesarean birth. Women with active herpetic vulvar lesions are advised to have cesarean birth rather than vaginal birth to minimize the newborn’s exposure. Infants with an infection should be separated from other infants in a nursery. Although transmission from this source is rare, women with herpes lesions on their face (herpes simplex I, or cold sores) need to be assessed before they hold their newborns to be sure lesions are crusted and, therefore, are no longer contagious. Healthcare personnel who have herpes simplex infections should not care for newborns until the lesions are crusted. Although facial herpes simplex lesions are probably caused by herpesvirus type 1, limiting contact does not seem excessive in light of the severity of HSV-2 disease. Urge a woman who is separated from her newborn at birth to view her infant from the nursery window and participate in planning care to aid bonding. HIV INFECTION HIV infection and AIDS can be caused by placental transfer or direct contact with maternal blood during birth. Because older children can also be exposed to this disease, the care of children with this infection is discussed in Chapter 42. Hyperbilirubinemia The term “hemolytic” is Latin for “destruction” (lysis) of red blood cells. A certain degree of lysis of red blood cells in the newborn results from the destruction of red blood cells by a normal physiologic process as the newborn breaks down excess red blood cells formed in utero (see Chapter 18). Hemolytic disease is present when there is excessive destruction of red blood cells, which leads to elevated bilirubin levels (hyperbilirubinemia). In the past, hemolytic disease of the newborn was most often caused by an Rh blood type incompatibility. Because the prevention of Rh antibody formation has been available for almost 50 years, the disorder is now most often caused by an ABO incompatibility. In both instances, because the fetus has a different blood type than the mother, the mother builds antibodies against the fetal red blood cells, leading to hemolysis of the cells, severe anemia, and hyperbilirubinemia. Rh Incompatibility In every pregnancy, a few red blood cells enter the maternal circulation. If the mother’s blood type is Rh negative and the fetal blood type is Rh positive, this introduction of fetal blood causes sensitization to occur and the woman to begin to form antibodies against the specific antigen (most commonly the D antigen). Few antibodies actually form this way during pregnancy, however. Most form in the woman’s bloodstream in the first 72 hours after birth because there is an active exchange of fetal–maternal blood as placental villi loosen and the placenta is delivered. Because of this surge in antibody formation after a pregnancy, in a second pregnancy, there will be a high level of antibody already circulating in the woman’s bloodstream. This will then act to destroy the fetal red blood cells beginning early in the next pregnancy if the new fetus is Rh positive, leading to the fetus being severely compromised by the end of that pregnancy. Rh incompatibility is not commonly seen today because if Rh-negative women receive Rho immune globulin (RHIG or RhoGAM) (passive Rh antibodies) within 72 hours after birth of an Rh-positive newborn, the process of antibody formation will be halted and sensitization will not occur. The possibility Rh incompatibility could exist, however, must be assessed for during pregnancy and again at birth because some women (especially those who received prenatal care in another country) may not have received RHIG following the birth or miscarriage of a former Rh-positive fetus. ABO Incompatibility In most instances of ABO incompatibility, the maternal blood type is O and the fetal blood type is either A or B type blood. Hemolysis can become a problem with a first pregnancy in which there is an ABO incompatibility because the antibodies to A and B cell types are naturally occurring antibodies or are present from birth in anyone whose red cells lack these antigens. Fortunately, unlike the antibodies formed against the Rh D factor, these antibodies are of the large (IgM) class and so do not cross the placenta. An infant of an ABO incompatibility, therefore, is not born anemic, as the Rh-sensitized child could be. Hemolysis of the blood begins with birth, when blood and antibodies are exchanged during the mixing of maternal and fetal blood as the placenta is loosened; destruction may continue for as long as 2 weeks. Interestingly, preterm infants do not seem to be affected by ABO incompatibility. This may be because the receptor sites for anti-A or anti-B antibodies do not appear on red cells until late in fetal life. Even in the mature newborn, a direct Coombs test may be only weakly positive because of the few anti-A or anti-B sites present. The reticulocyte count (immature or newly formed red blood cells) is usually elevated as the infant attempts to replace destroyed cells. Assessment Rh incompatibility of the newborn can be predicted by finding a rising anti-Rh titer or a rising level of antibodies (indirect Coombs test) in a woman during pregnancy. It can be confirmed by detecting antibodies on the fetal erythrocytes in cord blood (positive direct Coombs test) by percutaneous umbilical blood sampling (see Chapter 9) or at birth. The mother in this situation will always have Rh-negative blood, and the baby will be Rh positive. With Rh incompatibility, an infant may not appear pale at birth despite the red cell destruction that occurred in utero because the accelerated production of red cells during the last few months in utero compensates to some degree for the destruction. The liver and spleen may be enlarged from attempts to destroy damaged blood cells. If the number of red cells has significantly decreased, the blood in the vascular circulation may be hypotonic to interstitial fluid, causing fluid to shift from the lower to higher isotonic pressure by osmosis, resulting in extreme edema. Finally, the severe anemia can result in heart failure as the heart has to beat at a faster rate than normal to push the diluted blood forward. Hydrops fetalis is a Greek term that refers to a pathologic accumulation of at least two or more cavities with a collection of fluid in the fetus. Most infants do not appear jaundiced at birth because the maternal circulation has evacuated the rising indirect bilirubin level. With birth, progressive jaundice, usually occurring within the first 24 hours of life, will begin, indicating in both Rh and ABO incompatibility that a hemolytic process is occurring. The jaundice occurs because, as red blood cells are destroyed, indirect bilirubin is released. Indirect bilirubin is fat- soluble and cannot be excreted from the body. Under usual circumstances, the liver enzyme glucuronyl transferase converts indirect bilirubin to direct bilirubin. Direct bilirubin is water-soluble and combines with bile for excretion from the body through feces. In preterm infants or those with extreme hemolysis, the liver cannot convert all of the indirect bilirubin produced into direct bilirubin fast enough, so jaundice occurs. Normally, cord blood has a total serum bilirubin (TsB) level of 0 to 3 mg/100 ml. An increasing bilirubin level becomes dangerous if the level rises above 20 mg/dl in a term infant and perhaps as low as 12 mg/dl in a preterm infant because brain damage from bilirubin-induced neurologic dysfunction (BIND), a wide spectrum of disorders caused by increasingly severe hyperbilirubinemia ranging from mild dysfunction to acute bilirubin encephalopathy (ABE) (invasion of bilirubin into brain cells), can occur. A second concern that arises from excessive red blood cell destruction is that an infant is forced to use glucose stores to maintain metabolism in the presence of anemia. This can cause a progressive hypoglycemia, compounding the initial problem. A decrease in hemoglobin during the first week of life to a level less than that of the cord blood is a later indication of blood loss or hemolysis. Therapeutic Management Bilirubin levels in blood may be measured by either a blood draw (TsB) or by holding a transcutaneous meter against the infant’s skin (transcutaneous bilirubin [TcB]). The initiation of early feeding (urge mothers to breastfeed 8 to 10 times a day for the first 2 days), use of phototherapy, and exchange transfusion all may be measures necessary to reduce the TsB level in an infant affected by a blood incompatibility. In infants with severe hemolytic disease, the hemoglobin concentration can continue to drop during the first 6 months of life, or their bone marrow may fail to increase production of erythrocytes in response to continuing hemolysis so they need an additional blood transfusion to correct this late anemia. Therapy with erythropoietin to stimulate red blood cell production is also possible. The Initiation of Early Feeding Bilirubin is removed from the body by being excreted through the feces. Therefore, the sooner bowel elimination begins, the sooner bilirubin removal begins. Early feeding (either breast milk or formula), therefore, stimulates bowel peristalsis and helps to accomplish this. Phototherapy A fetus’s liver processes little bilirubin in utero because the mother’s circulation does this for the fetus. With birth, exposure to light is believed to trigger the liver to assume this function. Additional light supplied by phototherapy appears to speed the conversion of unconjugated (fat-soluble) into conjugated (watersoluble) bilirubin. Phototherapy exposes the infant to continuous specialized light such as quartz halogen, cool white daylight, or special blue fluorescent light. The lights are placed 12 to 30 in. above the newborn’s bassinet or incubator. Term newborns are generally scheduled for phototherapy when the TsB level rises to 10 to 12 mg/dl at 24 hours of age; preterm infants may have treatment begun at levels lower than this (Bhardwaj, Locke, Biringer, et al., 2017). Although the results of the therapy are mixed, the administration of intravenous immunoglobulin (IVIG) has been used in neonates with hemolytic disease in combination with phototherapy, especially in ABO incompatibility to try and extenuate the effect of phototherapy. Continuous exposure to bright lights by phototherapy may be harmful to a newborn’s retina, so the infant’s eyes must always be covered while under bilirubin lights. Commercial phototherapy masks or eye coverings must be used at all times when the infant is under phototherapy (with the use of bilirubin blankets, eye protection is not usually necessary if it is a full-term newborn). Check the eye covering/mask frequently to be certain it has not slipped. Infants are most apt to dislodge the eye covering when they cry as they wake for a feeding. Urge parents to respond quickly, therefore, if the infant is in their postpartum room to avoid eye damage and possible suffocation by the infant pushing the eye covering down over the nose. The stools of an infant under bilirubin lights are often bright green because of the excessive bilirubin being excreted as the result of the therapy. They are also frequently loose and may be irritating to the skin. Urine may be dark colored from urobilinogen formation. Monitor the infant’s axillary temperature to prevent him or her from overheating under the bright lights. Assess skin turgor and intake and output to ensure dehydration is not occurring from the warm environment. Infants receiving phototherapy should be removed from under the lights for feeding so they continue to have interaction with their mother. Remove the eye patches while the infant is out from under the lights for a period of visual stimulation. To prevent a lengthy hospital stay, infants may be discharged and continue therapy at home. Specialized fiber optic light systems incorporated into a fiber optic blanket also have been developed and are ideal for home care. The light generated by the blanket has the same effect on bilirubin levels as banks of overhead lights. The infant is undressed except for a diaper to protect the ovaries or testes and so as much skin surface as possible is exposed to the light. Two big advantages are that an infant can be held for long periods without interrupting the phototherapy, and eye patches are unnecessary. Parents need an explanation of the rationale for phototherapy and why their infant needs it. Although phototherapy has not been used long enough that long-term effects can be studied, there appears to be minimal risk to an infant from the procedure, provided the infant’s eyes remain covered and dehydration from increased insensitive water loss does not occur. Even though there is no evidence so far that infants who received phototherapy are at greater risk for developing skin cancer, all infants who receive phototherapy should (as should all infants) have sunscreen applied when they are in the sun and follow-up assessments in coming years to detect skin cancer that possibly could occur from the therapy (Oláh, Tóth-Molnár, Kemény, et al., 2013). Exchange Transfusion The use of intensive phototherapy in conjunction with hydration and close monitoring of serum bilirubin levels has greatly reduced the need for exchange transfusions. If this is done, small amounts (2 to 10 ml) of the infant’s blood are drawn from the infant’s umbilical vein and then replaced with equal amounts of donor blood. The therapy may be used for any condition that leads to hyperbilirubinemia or polycythemia. When used as therapy for blood incompatibility, it removes approximately 85% of sensitized red cells. It reduces the serum concentration of indirect bilirubin and can prevent heart failure in infants with severe anemia or polycythemia. A transfusion should be done under a radiant heat warmer to keep the infant warm during what can be a lengthy procedure to prevent energy expenditure from having to maintain body temperature. Donor blood must be maintained at room temperature, or hypothermia from the cold insult could result. Use only commercial blood warmers to warm blood, not hot towels or a radiant heat warmer, to avoid destroying red cells. The type of blood used for transfusion is O Rh-negative blood, even if an infant’s blood type is positive; if Rh-positive or type A or B blood were given, the maternal antibodies that entered the infant’s circulation would destroy this blood also, and the transfusion would be ineffective. If the baby will be transported to a regional center for the exchange transfusion, a sample of the mother’s blood should accompany the infant, so cross-matching on the mother’s serum can be done there. After a transfusion, closely observe the infant to be certain vital signs are stable and there is no umbilical vessel bleeding or inflammation of the cord if this was the transfusion site, which would suggest infection. Report any changes in vital signs. Monitor bilirubin levels for 2 or 3 days after the transfusion to ensure the level of indirect bilirubin is not rising again and that no further phototherapy or transfusion is necessary. Sudden Infant Death Syndrome Sudden infant death syndrome (SIDS) is a sudden unexplained death in infancy. It tends to occur at a higher than usual rate in infants of adolescent mothers, infants of closely spaced pregnancies, and underweight and preterm infants. Also prone to SIDS are infants with BPD, twins, Native American infants, Alaskan Native infants, economically disadvantaged Black infants, and infants of narcotic-dependent mothers. The peak age of incidence is 2 to 4 months of age. Although the cause of SIDS is unknown, in addition to prolonged but unexplained apnea, other possible contributing factors include: • Sleeping prone rather than supine • Viral respiratory or botulism infection • Exposure to secondary smoke • Pulmonary edema • Brainstem abnormalities • Neurotransmitter deficiencies • Heart rate abnormalities • Distorted familial breathing patterns • Decreased arousal responses • Possible lack of surfactant in alveoli • Sleeping in a room without moving air currents (the infant rebreathes expired carbon dioxide) Typically, affected infants are well nourished. Parents may report an infant had a slight head cold. After being put to bed at night or for a nap, the infant is then found dead a few hours later. Infants who die this way do not appear to make any sound as they die, which indicates they die with laryngospasm. Although many infants are found with blood-flecked sputum or vomitus in their mouths or on the bedclothes, this seems to occur as the result of death, not as its cause. An autopsy often reveals petechiae in the lungs and mild inflammation and congestion in the respiratory tract. However, these symptoms are not severe enough to cause sudden death. It is lear these infants do not suffocate from bedclothes or choke from overfeeding, underfeeding, or crying. Since the AAP made the recommendation to put newborns to sleep on their back, the incidence of SIDS has declined almost 50% to 60%. Other recommendations include the use of a firm sleep surface; breastfeeding; room sharing without bed sharing; routine immunizations; consideration of using a pacifier; and avoidance of soft bedding, overheating, and exposure to tobacco smoke, alcohol, and illicit drugs Although it was once thought having infants sleep with a fan in their room to keep air moving might decrease the incidence of SIDS, the AAP has noted that, currently, there is insufficient evidence to recommend the use of a fan as a SIDS riskreduction strategy. Parents have a difficult time accepting the death of any child. This can be especially difficult when it happens so suddenly and to an infant. In discussing the child, they often use both the past and present tense as if they are not yet aware of the death. Many parents experience a period of somatic symptoms that occur with acute grief, such as nausea, stomach pain, or vertigo. Parents should be counseled by someone who is trained in counseling at the time of the infant’s death; it helps if they can talk to this same person periodically for however long it takes to resolve their grief. The American Sudden Infant Death Syndrome Institute, listed at the beginning of the chapter, offers suggestions for counseling. Autopsy reports should be given to parents as soon as they are available (if toxicology tests are included in the autopsy, results will not be available for weeks). Reading that their child’s death was unexplained can help to reassure parents the death was not their fault. They need this assurance if they are to plan for other children. If there are older children in the family, they also need assurance SIDS is a disease of infants and the strange phenomenon that invaded their home and killed a younger brother or sister will not also kill them. If they wished the infant dead, as some children wish siblings were dead occasionally, they need reassurance their wishes did not cause the baby’s death. When another child is born, parents can be expected to become extremely frightened at any sign of illness in their child. They need support to see them through the first few months of the second child’s life, particularly until past the point at which the first child died. Some parents may need support to view a second child as an individual child and not as a replacement for the first child. A new baby born to a family in which a SIDS infant died can be screened using a sleep assessment as a precaution within the first 2 weeks of lifeor, if the parents’ level of anxiety is acute, before hospital discharge. The baby may then be placed on continuous apnea monitoring pending the results of the sleep assessment.