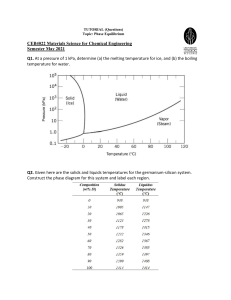
METALS • Metallic Solids, also simply called “metals” consist entirely of metal atoms. IMAGE SOURCE: Brown, 2011 IMAGE SOURCE: Brown, 2012 IMAGE SOURCE: Brown, 2011 • An alloy is a material that contains more than one element and has the characteristic properties of a metal. IMAGE SOURCE: Brown, 2012 CATEGORIES OF ALLOY 1. Substitutional alloy – when atoms of the solute in a solid solution occupy positions normally occupied by a solvent atom They are formed when the two metallic components have similar atomic radii and chemical-bonding characteristics. When two metals differ in radii by more than about 15%, solubility is generally more limited. IMAGE SOURCE: Brown, 2012 CATEGORIES OF ALLOY 2. Interstitial alloy - When the solute atoms occupy interstitial positions in the “holes” between solvent atoms. For an interstitial alloy to form, the solute atoms must have a much smaller bonding atomic radius than the solvent atoms. Typically, the interstitial element is a nonmetal that makes covalent bonds to the neighboring metal atoms. The presence of the extra bonds provided by the interstitial component causes the metal lattice to become harder, stronger, and less ductile. IMAGE SOURCE: Brown, 2012 CATEGORIES OF ALLOY 3. Heterogeneous alloy – when the components in the alloy are not dispersed uniformly. In general, the properties of heterogeneous alloys depend on both the composition and the manner in which the solid is formed from the molten mixture. IMAGE SOURCE: Brown, 2012 CATEGORIES OF ALLOY 4. Intermetallic compounds- are compounds rather than mixtures; hence, they have definite properHes and their composiHon cannot be varied. Unlike the atoms in substitutional and interstitial alloys, the different types of atoms in an intermetallic compound are ordered rather than randomly distributed. The ordering of atoms in an intermetallic compound generally leads to better structural stability and higher melting points than what is observed in the constituent metals. On the negative side, intermetallic compounds are often more brittle than substitutional alloys. CATEGORIES OF ALLOY IMAGE SOURCE: Brown, 2012 IMAGE SOURCE: Brown, 2012 IMAGE SOURCE: Brown, 2012 IMAGE SOURCE: Callister, 2007 IMAGE SOURCE: Callister, 2007 For a 40 wt% Sn–60 wt% Pb alloy at 150 C (300 F), (a) What phase(s) is (are) present? (b) What is (are) the composition(s) of the phase(s), in terms of mass fraction? IMAGE SOURCE: Callister, 2007 REFERENCES Brown, T.L., Lemay Jr., H.E., Bursten, B.E., Murphy, C.J., and Woodward, P.M. (2012) Chemistry: The Central Science, 12th Ed., USA: Pearson Education, Inc. Brown, L.S. and Holme, T.A. (2011) Chemistry for Engineering Students, 2nd Edition, USA, Brooks/Cole, Cengage Learning Callister Jr., W., (2007) Material Science and Engineering: An Introduction, USA, John Wiley & Sons