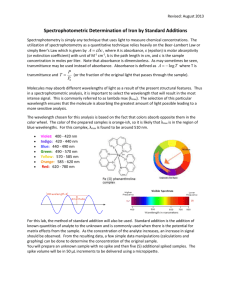
INSTRUMENTAL ANALYSIS METHODS, 2014. Laboratory work No. 1 Photometric determination of chromium (III) with complexone III (EDTA) Chromium (III) complexonate Thermodynamic stability constant of chromium (III) complexonate CrY- is 1023.4. Because of inertness of chromium (III) aqua complex, formation of Cr(III) complexonate in aqueous media and under ambient temperature is very slow. In 100°C exchange with ligands becomes rapid and after several minutes blue-purple coloured chromium (III) complexonate is made. [Cr(H2O)n]3+ + H2Y2- + 2CH3COO- → CrY- + 2CH3COOH + nH2O If chromium (III) complexonate is made in solutions with media pH 4-5, its absorbance maximum is at 540nm (Figure 1). Molar absorbtion coefficient is only 140. Decreasing pH of the media it gets even lower. To ensure optimal pH value, acetate buffer solution with ratio of components 1:1 is used so pH=pKa=4.8. Figure 1. Absorbtion spectrum of chromium (III) complexonate Spectrophotometer / Photometer Two single beam devices will be employed: Spectrophotometer Jenway 6300 with 1cm cuvette and photometer KFK2. The principial scheme is shown in figure 2. Figure 2. Optical scheme of the spectrophotometer 1 Main difference between both instruments is in the way how monochromatic light is obtained. In spectrophotometer Jenway 6300 diffraction grating is used to obtain light with defined wavelenght. In photometer KFK2 optical filters are used to give monochromatic light. (Please evaluate the wavelength precision of both monochromators). Diffraction grating is an optical component with a periodic structure, which splits and diffracts light into several beams travelling in different directions. The directions of these beams depend on the spacing of the grating and the wavelength of the light so that the grating acts as the dispersive element. Absorbtive filters absorb some wavelengths of light while transmitting others. Using data from table 1.1. we can find filter which will serve as monochromator. Table 1. Absorbtive filters Wavelenght region absorbed, nm 400 – 435 435 – 480 480 – 490 490 – 500 500 – 560 560 – 580 580 – 595 595 – 600 650 – 750 Colour of light absorbed Violet Blue Blue-green Green-blue Green Yellow-green Yellow Orange Red Complementary colour transmitted Yellow-green Yellow Orange Red Purple Violet Blue Blue-green Green-blue Monochromatic light band has shape of symmetrical peak with parameters as nominal wavelenght, maximum throughput and effective bandwidth (figure 3). Figure 3. Monochromatic light band 2 Typical spectra of photometer filter set is shown in figure 4. Figure 4. Transmittance of absorbtive filters (KFK-2) The principle of light absorbance measurment Photometers and spectrophotometers are used to measure the absorbance of coloured sample solutions againtst the absorbance of zero standard sample. Using the zero sample absorbance is set to 0, then the absorbance of analytical sample is measured. Task 1. Obtain data for calibration graph. Construct calibration plot, find the equation of the trendline and coefficient of corellation. Evaluate the quality of the trendline. 2. Find the mass (g) of chromium (III), which is in 100mL flask given by the lab assistant. Experimental Connect instrument to mains, turn on. Preparation of solutions Dilute analytical sample in 100mL volumetric flask (given by the lab assistant) with deionized water up to the mark and mix well. With pipette transfer 10mL of obtained solution into 100mL beaker (Marked ‘’An’’). With volumetric pipette add 10mL of deionized water, 5mL of acetate buffer solution and 10mL of complexone III solution. Using burette transfer 20mL of chromium (III) standard solution (γ=0.002g/mL) into 50mL volumetric flask, dilute with deionized water up to the mark and mix well. Label six 100mL beakers with inscriptions 0, 2, 4, 6, 8 and 10. 3 Using volumetric pipette transfer appropriate volume of previously prepared chromium (III) standard solution into labelled beakers: 2mL → beaker #2 4mL → beaker #4 6mL → beaker #6 8mL → beaker #8 10mL → beaker #10 Then add deionized water: 20mL → beaker #0 18mL → beaker #2 16mL → beaker #4 14mL → beaker #6 12mL → beaker #8 10mL → beaker #10 To each beaker add 5mL of acetate buffer solution and 10mL of complexone III solution. Heat all 7 beakers to a boil and continue boiling for another 5 minutes and allow them to cool down. Label seven 100mL volumetric flasks with inscriptions 0, 2, 4, 6, 8, 10 and ‘’An’’. Transfer solutions from beakers to respective volumetric flasks. Wash each beaker with deionized water and transfer it to respective flask (don’t pour it out!). Repeat washing for several times to be sure that chromium (III) complexonate is completely (quantitatively) transfered to 100mL flasks. Then dilute solutions in volumetric flasks with deionized water till the mark and mix them well. Measurment of light absorbance Absorbtion measurment of chromium (III) complexonate solution is performed against the 0 sample using 1cm cuvette (KFK2) and 5cm cuvette (Jenway 6300). Start measurment with the more diluted standard sample. Before you do each measurment, flush cuvette with current solution. When you have finished with all the standards, flush cuvette with deionized water, then with analytical solution ‘’An’’, then fill cuvette with analytical solution and perform the measurment. Spectrophotometer Jenway 6300 1. Connect the instrument to the mains, turn on and allow to condition for 15 minutes. 2. Choose the wavelength and measurment mode (T, ABS, CONC). 3. Open cuvette compartment, insert cuvette with ‘’0’’ sample solution, close compartment and press CAL button. Absorbtion value is set to ABS=0. 4. Remove cuvette with ‘’0’’ solution, flush and fill it with next standard solution put cuvette into holder and close compartment. Read the absorbance value. 1. 2. 3. 4. 5. Photometer KFK-2 Connect the instrument to the mains, turn on and allow to condition for 15 minutes. Set the appropriate light filter (controlling the filter position of 540nm mark). Set sensitivity ‘’чувствительность’’ to position 1 (minimal), turn tuner knob ‘’установка’’ to the left till the end. Open cuvette compartment and instert cuvette with ‘’0’’ solution, using sensitivity knob and tuner set transmittance to 100. Into second slot put cuvette with Cr(III) containing solution. Using handle below cuvette compartment replace both cuvettes so the sample cuvette gets under the light beam. Read the transmittance on the upper scale or absorbance on the lower scale. 4 University of Latvia Faculty of Chemistry ________________________ Name Surname Laboratory work No. 1 Photometric determination of chromium (III) with complexone III (EDTA) 1. Theoretical background Equation of hydrated chromium (III) ion reaction with complexone III (show oxidation state of all atoms): 2. Equipment Draw optical scheme of KFK-2 with explanations (attach on separate sheet). Motivate the wavelength selection: 3. Preparation of solutions Calculate mass concentration of chromium (III) ions in solution which is obtained when 20mL of chromium (III) standard solution (γ=2mg/mL) are diluted with deionized water in 50mL volumetric flask. Show all members and units! Give equation to calculate chromium (III) mass concentration in labelled 100mL volumetric flasks ‘’2’’, ‘’4’’, ‘’6’’, ‘’8’’, ‘’10’’. Show all members and units! Cromium (III) ion mass concentrations are: Flask ‘’2’’: γ= Flask ‘’4’’: γ= Flask ‘’6’’: γ= Flask ‘’8’’: γ= Flask ‘’10’’: γ= 5 4. Measurments Enter calculated data and obtained measurments in table 1. Solution Table 1 Mass concentration and absorbance of Cr (III) solutions Chromium (III) Absorbance, 1cm Absorbance, 5cm concentration γ, μg/mL cuvette cuvette ‘’2’’ ‘’4’’ ‘’6’’ ‘’8’’ ‘’10’’ An 5. Calculation of results Use MS Excell software to construct two calibration graphs. Put chromium (III) mass concentration on x-axis and absorbance on y-axis. If the graph shows experimental points that are very different from those points that form a straight line, then they are gross errors and may be excluded. Use MS Excell to find the equation of calibration curve y=A+Bx: A1cm cuvette = B1cm cuvette = A5cm cuvette = A5cm cuvette = Use equation of calibration curve to calculate chromium (III) concentration in analytical solution using both 1 and 5cm cuvettes: γ1cm cuvette = γ5cm cuvette = Compile the equation, enter values and units, and calculate the mass of chromium (III) ions in analytical solution: 6. Job quality rating True mass of iron ions: Absolute error: Relative error: Conclusions about the accuracy of the work. Compare obtained error to the method-specific random deviation values. Give conclusions whether the work is vitiated by systematic error. On horizontal line mark true and found chromium (III) ion mass. Mark lower and higher limit of confidence interval with the brackets: 6 Assess the obtained absorbance values, explain why absorbance in 1cm cuvette is smaller than in 5cm cuvette: Is there a need to check (calibrate) the volume of volumetric glassware? Motivate Your answer with comparison of method specific random errors to uncertainty of class B glassware. Mācību metodiķa paraksts par darba vietas pieņemšanu: Work performance date: 2014.___.____________ Student’s signature: Work counted: 2014. ___.___________________ Teacher’s signature: 7 Appendix 1 1. Chemicals Cr2(SO4)3 · 6H2O Cr3+, c=2mg/mL CH3COONa, c=0,1mol/L Complexone III, 5% solution 2. Glassware 100mL volumetric flasks, 8pcs 100mL beakers, 7pcs 10mL Mohr pipettes, 3pcs 50mL volumetric flask 10mL graduated pipette 25mL measuring cylinder Funnel 3. Equipment Dispenser Jenway 6300 KFK-2 8