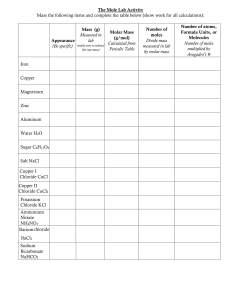
Accepted Manuscript Title: Review on the significance of chlorine for crop yield and quality Author: Christoph-Martin Geilfus PII: DOI: Reference: S0168-9452(17)30770-7 https://doi.org/10.1016/j.plantsci.2018.02.014 PSL 9757 To appear in: Plant Science Received date: Revised date: Accepted date: 16-8-2017 29-12-2017 13-2-2018 Please cite this article as: Christoph-Martin Geilfus, Review on the significance of chlorine for crop yield and quality, Plant Science https://doi.org/10.1016/j.plantsci.2018.02.014 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. Review on the significance of chlorine for crop yield and quality Christoph-Martin Geilfus IP T C.-M. Geilfus; Controlled Environment Horticulture, Faculty of Life Sciences, Albrecht Daniel Thaer-Institute of Agricultural and Horticultural Sciences, Humboldt-University of SC R Berlin, Albrecht-Thaer-Weg 1, 14195 Berlin, Germany. E-mail: geilfusc@hu-berlin.de. U Phone: +49(0) 30 2093 46475 A CC E PT ED M A N Graphical abstract 1 Highlights Cl--salinity induces dysfunctions that hamper quality formation in crops. Starch partitioning, nutrient uptake, protein biosynthesis or photosynthesis are impaired. Action is needed to stabilize yields and quality under Cl--salinity. IP T Thus, dietary quality of harvests is lowered by high tissue concentration of Cl-. SC R Restricted Cl--xylem loading or NO3-fertilization limit Cl- accumulation. Abstract U The chloride concentration in the plant determines yield and quality formation for two N reasons. First, chlorine is a mineral nutrient and deficiencies thereof induce metabolic A problems that interfere with growth. However, due to low requirement of most crops, M deficiency of chloride hardly appears in the field. Second, excess of chloride, an event that occurs under chloride-salinity, results in severe physiological dysfunctions impairing both ED quality and yield formation. The chloride ion can effect quality of plant-based products by PT conferring a salty taste that decreases market appeal of e.g. fruit juices and beverages. However, most of the quality impairments are based on physiological dysfunctions that arise CC E under conditions of chloride-toxicity: Shelf life of persimmon is shortened due to an autocatalytic ethylene production in fruit tissues. High concentrations of chloride in the soil can increase phyto-availability of the heavy metal cadmium, accumulating in wheat grains A above dietary intake thresholds. When crops are cultivated on soils that are moderately salinized by chloride, nitrate fertilization might be a strategy to suppress uptake of chloride by means of an antagonistic anion-anion uptake competition. Overall, knowledge about proteins that catalyse chloride-efflux out of the roots or that restrict xylem loading is needed to engineer more resistant crops. 2 Abbreviations: Chloride, Cl−. Keywords: Chloride, Photosynthesis, Osmoregulation, Tonoplast-type H+-ATPase, BlossomEnd Rot, Crops, Yield, Quality, Fungal Infections. Introduction 2. Cl− as nutrient Osmo- and turgor-regulation 2.2 Elongation growth and tonoplast-type H+-ATPase 2.3 Rhythmic movements 2.4 Action potentials as signals? 2.5 Enzyme activities 2.6 Photosynthesis M A N U 2.1 ED Cl− excess constrains yield formation Inhibition of photosynthetic capacity 3.2 Retardation of root and tuber growth 3.3 The Cl−-sensitive potato 3.4 Antagonistic anion-anion effects PT 3.1 CC E 3. SC R 1. IP T Tables of Contents A 4. 5. Dysfunctions under Cl−-toxicity and implication for food quality and safety 4.1 Protein biosynthesis 4.2 Blossom-end rot 4.3 Ethylene production 4.4 Enhance cadmium uptake 4.5 Loss of quality of fruit juices and beverages in response to Cl−-toxicity Cl− fertilization for fungal infection management 3 6. Concluding remarks 7. Outstanding questions 1. Introduction Chloride (Cl−) is a nutrient and beneficial ion for higher plants [1,2]. The concentration of Cl− IP T in the soil and its bulk solution mainly depends on bedrock material, depositions of airborne Cl− via e.g. precipitation, or on inputs through fertilization or irrigation with Cl−-containing SC R waters [3]. The average Cl− concentration in soils is 0.10 g kg-1 soil [4]. Cl− is barely adsorbed to soil particles [5] and since it is highly water soluble [6], it is very mobile in soil solution. The monovalent anion travels with soil water towards the root where it is taken up into the U plant, a process that correlates well with water flow [7]. In the roots, Cl− moves N predominantly via the symplastic pathway radially towards the vascular stele [8]. Before Cl− A is acropetally translocated via xylem to the shoot, it needs to enter the root xylem. In M Arabidopsis, this is partially mediated by the stelar-localized anion/H+ cotransporters protein ED NPF2.4 that facilitates the loading of Cl− into the root xylem [9]. For more molecular details regarding Cl− uptake into the root cells or xylem loading and unloading, the reader is referred PT to recent works [10,11]. After Cl− is released in the shoot, it can be distributed via the phloem [12], reaching the cytosol for e.g. storage in the vacuole [13] or incorporation in the oxygen- CC E evolving complex of the photosystem II [14]. The Cl− concentration in the shoots of all major glycophytic crop plants ranges from 1-20 mg g dw-1 [15-19]. This depends on species, A genotype and the level of Cl− in the environment [20]. Under conditions of excess of Cl− in the environment, tissue concentrations can exceed toxic levels in our crops, whereupon yield and quality are reduced. Not much is known about how toxic level of Cl− are mechanistically linked with processes that impair quality formation. There is a dearth of published data and the few information are scattered. Here, the historic and the recent literature is reviewed and novel hypotheses are developed that led to the 4 suggestion of novel experiments that are urgently needed to bring the topic forward. The first part in this review is meant to provide a survey about functions of Cl− as nutrient. Then, aspects related to the accumulation of excess of Cl− are discussed with special emphasis on toxicity-related constraints for quality formation. Recommendations to produce yields with a 2. IP T high quality are worked out. Cl− as nutrient SC R Most glycophytic crop plants (plants that will only grow in soils with a low content of salts) contain 1-20 mg Cl− g dw-1 [15,16]. However, deficiency symptoms are observed only at much lower concentrations. Thresholds for deficiency strongly depend on the crop species. U Rice (Oryza sativa) is deficient at Cl− concentrations below 3 mg g shoot dw-1, wheat A N (Triticum aestivum) and barley (Hordeum vulgare) at concentrations below 1.2-4.0 mg g dw-1, spinach (Spinacia oleracea) and lettuce (Lactuca sativa) at concentrations below 0.14 mg g M dw-1, and maize (Zea mays) when Cl− falls below a concentrations of 0.05-0.11 mg Cl− g dw-1 ED [3]. By assuming a minimal requirement of 1 mg Cl− g dw-1, it was calculated that rainfall at inland sites would deposit the required Cl−, which amounts to 4-8 kg ha-1 [16]. Thus, despite PT rare exceptions [21], a Cl− deficiency in the field is considered an unlikely event that might only be observed when plants with a high Cl− requirement such as palm trees (Elaeis spp.) (6 CC E mg Cl− g leaf dw-1) or kiwifruit (Actinida deliciosa) that require at least 60 µM g-1 leaf DW [22] are cultivated in highly leached soils without any Cl− inputs (Marschner, 2011). For A instance, it was calculated that one ton of dried tomato plants requires only a minimum of 200 grams of chlorine [1]. However, lack of Cl− can be studied under laboratory conditions in hydroponic cultivation systems and these studies revealed that Cl− deficiency symptoms include leaf growth reduction and wilting, which can be followed by chlorosis and necrosis [1]. Although Cl− has a high mobility in the plant, Cl− deficiency symptoms are generally first 5 observed in young leaves [23]. Functions of Cl− as a micronutrient or beneficial element will be discussed next. 2.1 Osmo- and turgor-regulation IP T Cl− is a micronutrient [24] but, during its function in osmo- and turgor-regulation, it can accumulate in the vasculature, in guard cells or in expanding tissues reaching, at some SC R instances, a final concentration of up to 150 mM in the vacuole [2,16]. Here, Cl− serves as an osmoticum [25,26] that drives the water flow. In result, turgor increases which facilitates compound movement [27], effects source-sink partitioning and influences turgor-driven U movements. In the guard cells during stomatal aperture adjustments, Cl− has a similar function N as malate. It counterbalances influx of potassium during stomata opening [28]. Accumulation A of Cl− together with potassium and malate decreases guard cell water potential in vacuoles, M whereupon water streams in, causing the guard cells to swell [29,30]. In guard cells, this ED potassium-counterbalancing role is dominantly exercised by malate [31], but Cl− is also functional when malate is present [32-34]. It was found that the vacuolar chloride channel PT AtALMT9 controls the stomatal aperture via facilitating Cl− fluxes, whereby this channel is activated by malate [13]. In agreement, it was shown for field bean (Vicia faba) that Cl− is CC E sequestered in the vacuole of guard cell through the action of a kinase-dependent vacuolar chloride channel, whereby this Cl− influx is required for stomatal opening [35]. Based on the A general relevance for osmo-regulation and control of stomatal aperture, a debate has emerged over the assumption that Cl− is relevant for water- and nitrogen use efficiencies in crops [2,11]. 2.2 Elongation growth and tonoplast-type H+-ATPase 6 Cl− stimulates the activity of the tonoplast-type H+-translocating ATPase [36,37]. By this, it contributes to the maintenance of the pH gradient between the slightly alkaline cytosol (pH = 7.2-7.5; [28]) and the acidic vacuole (pH = 5.0-5.5; [38,39]). This establishes a positive membrane potential over the tonoplast [40], a potential that can reach +86 mV inside the vacuole of the bubble algae (Valonia ventricosa) [41]. This difference of membrane potentials IP T favours the intrusion of anions (e.g. nitrate) into the vacuole, where they serve as an osmoticum. In consequence, water accumulates within the vacuoles and turgor pressure SC R increases, which is thought to give rise to turgor-driven cell expansion in shoot and root tissue [42,43,44]. In this way, Cl− is thought to be instrumental for facilitating elongation growth via effects exerted through the V-type H+-ATPase on turgor [45]. Of note, the establishment of a U positive membrane potential inside the vacuole wills also favours the influx of Cl− along its N electrical gradient into the vacuole. The observation that the addition of 10 mM Cl− into a A nutrient solutions of corn (Zea mays) and tomato (Lycopersicon esculentum) had a positive M effect on growth [46], together with the fact that vacuoles can accumulate the osmotically ED active Cl−, gives rise to the idea that Cl− tolerant crops such as chard (Beta vulgaris subsp. vulgaris) or clover (Trifolium repens) could be fertilized by moderate doses of Cl− in order to PT promote expansion growth. What is needed are experiments with increasing Cl− supply to test for a dose that increases turgor-driven expansion growth without inducing cellular damages CC E associated with Cl−-toxicities. The idea that uptake of Cl− favours expansion growth is supported by the finding that the ꞌacid-growthꞌ-controlling phytohormone auxin [47] A stimulates Cl− uptake by oat coleoptiles [48]. Moreover, it was found that a Cl− fertilization at a rate of 44.83 kg ha-1 increases yield of corn and grain sorghum (Sorghum vulgare) when applied to a soil that contains less than 22.42 kg ha-1 (0 - 60-cm depth) [49]. The fertilization of winter wheat with Cl−, given as NH4Cl, markedly increased fresh weight yield and kernel weight over those yields obtained by (NH4)2,SO4 fertilization (sulfate was sufficiently added 7 to the NH4Cl-treated plots). Cl− was discussed to cause these yield increases by increasing leaf turgor potential for the facilitation of expansion growth [50]. V-ATPases are also expressed in the endomembrane systems of the endoplasmic reticulum or the Golgi vesicles [50]. Here, via the generation of potentials across the membranes, VATPase are implicated in a wide range of ion and metabolite transport processes and in IP T secretory and endocytic trafficking [51,52]. In other words, it can be assumed that Cl− is indirectly involved in all those processes but it is an open question what will happen if the SC R cellular Cl− homeostasis cannot be maintained under conditions of e.g. Cl−-toxicity. Are VATPases-facilitated trafficking processes influenced? Rhythmic movements U 2.3 N The rhythmic leaf movement of the pink silk tree (Albizzia julibrissin) is mediated by A potassium influxes that are counterbalanced by Cl− influxes [53]. Influx of these ions M increases turgor by decreasing the osmotic potential, which drives water influx. Besides ED functions in nastic movements, pulvinar movements are also influenced by Cl − activities. For 2.4 PT more insights, the reader is referred to a recent work [2]. Action potentials as signals? CC E There is some indication that Cl− fluxes between subcellular compartments generate action potentials [54] that are suggested to be relevant for cellular communication [55,56]. More A work is required to verify this and it would be interesting to know if this could be related to signalling the onset of Cl−-salinity. During Cl−-salinity, cellular Cl−-concentrations can accumulate up to the millimolar-range, a concentration that might be sufficient to generate action potentials when channelled across (endo)membranes. 2.5 Enzyme activities 8 The stimulating function of the activity of the tonoplast-type H+-ATPase was discussed above. Besides, a link between Cl− and glutamine utilization for asparagine synthesis was vaguely reported [57]: In the annual yellow-lupin (Lupinus luteus) Cl− acts as an allosteric activator that increases the affinity of the glutamine-dependent asparagine synthetase to glutamine by up to 50-fold. A glance to mammalian- or bacterial systems reveals the IP T existence of more of such interactions. Cl− is known to regulate the activity of the angiotensin-converting enzyme in human somatic cells [58] or the activity of the alpha- SC R amylase in the proteobacteria Alteromonas haloplanctis [59]. It is an intriguing observation that Cl− can act as allosteric enzyme activator. Upon binding, Cl− increases activity of the αamylase from the bacteria Pseudoalteromonas haloplanktis via positive allosteric modulation. U Cl− binding is not mandatory for the proper enzyme folding but enhances the enzyme N reactivity and the structural stability of this bacterial α-amylase [60]. It is not fully resolved A whether Cl− has such an in vivo effect on the activity of α-amylase in plants. There is only a M very early study on potato (Solanum tuberosum) that reports that Cl− accelerates and stabilizes ED the activity of the α-amylase [61]. The Starch content is a key quality determinant for potatoes. Thus, it remains to be elucidated as to whether starch content in potatoes can be PT (substantially) increased by a slight supply of Cl− (the reader is referred to chapter 3.3 where the sensitivity of potato to Cl− is discussed). Photosynthesis CC E 2.6 Cl− has a major function in the photosynthetic light reaction. In the year 1946, a Cl−-depended A stimulation of the Hill reaction was demonstrated [62]. It was then reasoned that Cl− indirectly stimulates the oxygen evolution system by protecting chloroplastic components from being destroyed by light [63]. This led to the suggestion that Cl− has a structural function in the photosynthetic electron transport chain during the Hill reaction [64]. In vitro experiments on isolated chloroplasts from sugar beet (Beta vulgaris) have shown that Cl− content can be decreased by washing them with the Cl− chelator ethylenediaminetetraacetic acid [65]. When 9 Cl− was added again, a 10-fold stimulation of the photosynthetic activity was observed. Later, it was found that Cl− protects peptides of the photosystem II from dissociation and it was concluded that Cl− has a structural role, which is to stabilize polypeptides from this photosystem, thereby enabling the oxidation of water [66]. Crystal structure analysis of the oxygen-evolving complex of the photosystem II indicated a role of Cl− in the structural IP T organization of the Mn4CaO5-cluster [14]. The finding that Cl− binding sites near this Mn4CaO5-cluster are exposed to the luminal bulk solution gave rise to the idea that Cl− is an SC R integral compound of structures that function either as a proton exit channel or water inlet channel [14]. It was proposed that the binding of Cl− near the oxygen-evolving complex avoids the formation of a salt bridge between aspartic acid and lysine [59]. The formation of U such a bridge might otherwise result in a conformational shift that lowers the pKa of this N residue, being unfavourable for the ability to accept protons during the S-state cycle of the M Cl− excess constrains yield formation ED 3. A water oxidizing process. Cl− toxicity can result in chlorotic discolorations and later in necrotic leaf edges. Growth of PT glycophytes is sizeably reduced [67]. Visual toxicity symptoms can start with chlorosis at the leaf edges, which might precede leaf tip burn [68] or in extreme situations leaf abscission. CC E However, such Cl−-injuries are not only a thread to global food security, they are also problematic for ornamental crops such as roses (Rosa ×fortuniana or R. odorata), because A they reduce the aesthetic appearance and thus consumer acceptance and marketable yield [69]. High concentration of Cl− in the tissue, particular in the cytosol or chloroplast, are thought to limit the growth of a wide range of glycophytic plants [46,70-74]. Reasons for this are not fully resolved. It can be differentiated between Cl−-sensitive crops such as bird's-foot trefoil [75] (Lotus corniculatus,), Citrus rootstocks [76], or grapevine (Vitis vinifera, [74]), that show toxicity symptoms in the shoots at concentrations ranging from 4-7 mg g dw-1, whereas less10 sensitive crops show symptoms only at concentrations between 15-33 mg g dw-1 [3]. Sugar beet (Beta vulgaris) has halophytic ancestors (halophyte; plant that survives to reproduce when salt concentration is around 200 mM NaCl or more; [77,78]) and, here, Cl− is only toxic at concentrations higher than 50 mg g dw-1. Of note, halophytes such as grey mangrove (Avicennia marina) require much higher Cl− levels for photosynthetic light reaction [79]. IP T Thus, halophytes accumulate more Cl− than glycophytes when cultivated with less than 1 mM Cl− [80]. SC R A descriptive modelling approach was used to calculate that grain yield will be reduced by 10% when the subsoil Cl− concentration reaches 490 mg kg-1 (calculated for chickpea production; Cicer arietinum), 662 mg kg- 1 (durum wheat; Triticum durum), 854 mg kg-1 U (bread wheat, Triticum aestivum), 980 mg kg-1 (canola; Brassica spp.) or 1012 mg kg-1 N (barley, Hordeum vulgare) [81]. These data are based on a vertisol, a soil type that is A dominated by the phyllosilicate clay montmorillonite [82]. Thresholds are anticipated to be M lower in loamy soils that adsorbs less Cl−. Physiological reasons of the underlying ED dysfunctions have not been very well resolved and toxicity symptoms are deceptive because 3.1 PT visual appearance can be similar under conditions of other nutrient deficiencies [3]. Inhibition of photosynthetic capacity CC E Photosynthetic capacity of glycophytes is affected by the excessive accumulation of Cl− in the chloroplasts [73]. In Cl−-stressed field bean (Vicia faba L.), leaf chlorotic symptoms were A ascribed to high chloroplastic Cl− concentrations being responsible for a reduction in chlorophyll content [83]. In accordance, it was reported about a chlorophyll degradation in Cl−-stressed field bean that was associated with a concomitant reduction in photosynthetic capacity, quantum yield and growth [84]. This was discussed to be the result of a Cl−-induced disturbance of the structural organization of the photosystem II that is likely to occur under Cl−-salinity since the chloroplast envelope has a high permeability for Cl− [85]. Cl− 11 concentrations ranging from 250-300 mM have been found in the stroma of the chloroplasts in Cl−-stressed common bean (Phaseolus vulgaris, [86]). A disturbance of the photosynthesis by too high concentrations of Cl− in the chloroplast is likely to be a reason why plants show stunted growth. A lack in assimilates and energy equivalents (i.e. ATP and NADH+H+) might account for a reduced anabolic activity, limiting growth of glycophytes. If this is true, Cl−- IP T toxicities may also impair the quality of crops by lowering the carbohydrate content. Carbohydrate content is a major quality attribute of our starchy crops. Besides cereals, species SC R such as banana (Musa ×paradisiaca), pea (Pisum sativum), lentil (Lens culinaris) or cassava (Manihot esculenta) belong to the carbohydrate-rich crops that are globally important for providing an energy-rich diet to people in developing countries. What is needed are U investigations that estimate if and how Cl−-toxicities will reduce the energy density of those A Retardation of root and tuber growth M 3.2 N foods and feeds. ED In contrast to aspects of Cl−-toxicity in the shoot, literature bearing on mechanistic reasons for linking Cl−-toxicity with root development is barely available. In Cl−-stressed pea (Pisum PT sativum L.), the respiration in root tips has been found to be depressed because of a shift from the glycolysis to the pentose phosphate pathway, a shift thought to interfere with the energy CC E supply [87]. In another study, Cl−-salinity has been reported to dampen the root growth of avocado (Persea americana L) resulting in problems regarding water supply [88]. The A mechanisms behind this root growth retardation were never studied with a focus on Cl−associated effects. The addition of 100 mM NaCl to the roots of Arabidopsis that grew in agar media resulted in a quiescent phase in lateral roots that retarded growth for several days [89]. This growth suppression is controlled by abscisic acid signalling at the endodermis, which serves as an important site for sensing NaCl-stress. These results are very thrilling, but the experimental design does not allow concluding as to whether this retardation in growth was 12 induced either by the sodium, by Cl−, or by the osmotic stress component of NaCl-stress. Further work is appreciated to advance our understanding of how toxic Cl− concentrations in the soil are mechanistically linked to physiological dysfunctions leading to root growth reductions. Knowledge with regard to this would be significant because many of our starchy food plants are root and tuber crops. These include besides potato and cassava (Manihot IP T esculenta) also bulb and tuberous Allium and Beta vulgaris subspecies vegetables such as 3.3 SC R onion, garlic or beets. The Cl−-sensitive potato Caution is needed when Solanum tuberosum is fertilized with Cl−-containing mineral or U organic fertilizers. Fertilization of 400 kg Cl− ha-1 via KCl decreases tuber yield, retards crop N growth and slows down emergence [90]. However, an application of 400 kg of potassium A would be higher than required (180-240 kg K+ ha-1 for table potato; 110-180 kg K+ ha-1 for M starch potato). However, since the addition of 210 kg total N ha-1 year-1 via pig slurry ED amendments (finishers) carries a concomitant input of > 450 kg Cl− ha-1 year-1 [91], the abovedescribed scenario might become true. The physiological reasons for the tuber yield reduction PT are unclear. Besides the possibility of a Cl−-induced quiescent phase in roots or maybe stolons, as speculated in section 3.2, tuber yield reduction could be the result of an impaired CC E uptake of nitrate or an inhibited photosynthesis [92]. High amounts of Cl− (e.g. 6.1 g Cl− per pot) decrease leaf chlorophyll content and result in a reduction of the photosynthesis [93]. In A result, the total sum of carbohydrates decreases in the leaves while starch content increases. The latter is indicative for an impaired carbohydrate partitioning. The leaves that accumulated starch grew better compared to the tubers [93,94]. Trying to explain this divergent growth between the shoot and the tubers, a hypothesis was expressed [94]: It was suggested that Cl− accumulates in the vacuoles of the leaf cells, which decreases the leaf osmotic potential, driving water inflow in shoots. Shoot turgor pressure increases, ultimately favouring turgor13 driven cell expansion and shoot growth. The growing shoot is then thought to act as a strong sink for assimilates, having a sink strength that dominates over, or at least competes with, the sink strength of the tubers. Assimilates such as starch are preferably delivered into the expanding tissues of the shoot, and not to the tubers. This reduction of the carbohydrate delivery to the tubers may delay tuber development and growth. This theory has to be tested 14 C tracer experiments to elucidate carbon translocation rates and allocation IP T by using in situ patterns between shoot and tuber. For this, leaves have to be labelled with 14 [C]-sugar SC R molecules that are brushed on abraded zones of leaves. Partitioning of labelled sugar molecules between donor leaves and sink organs (root, youngest immature leaves) can be monitored. Different Cl−- and sodium-accompanying counter ions have to be used that allow N Antagonistic anion-anion effects A 3.4 U testing for Cl−-specific effects. M Antagonistic ion-ion uptake interactions are attributable for nutrient deficiency that are ED induced by Cl−-salinity. When the external Cl− concentrations is very high, an antagonism between Cl− and nitrate uptake has been reported in Brassica rapa [95] and Citrus [96]. This PT phenome may be based on an inhibition of nitrate transporters, as shown for the Arabidopsis thaliana nitrate transporter 1/peptide transporter NPF7.3. NPF7.3 is located in the root CC E pericycle cells where it catalyzes xylem loading of nitrate [97]. However, when concentration of Cl− increase in the environment and subsequently in the roots, it was observed that nitrate A concentration increase in the roots but decrease in the shoot, while increasing amounts of Cl− are loaded into the xylem being delivered to the shoot [98]. It is thought that NPF7.3 leaks Cl− at too high concentrations because NPF7.3 cannot discriminate nitrate over Cl−, by this displacing nitrate from being quantitatively taken up [10,11]. Such a competitive uptake effect is thought to induce a lack of nitrogen in the shoot, finally hampering growth and yield [2,67] as shown for wheat (Triticum aestivum L.; [99]), grapevine (Vitis sp.; [100]), or tomato 14 (Solanum lycopersicum; [101]); with exception for Zea mays L. [102]. For the tomato (Lycopersicon esculentum Mill), it was shown that the nitrate dose needs to be increased to ensure an adequate N supply [103] under conditions of excess of external Cl−. This is an interesting observation that is worth being tested as strategy to cope with the problem of excess accumulation of Cl− in the shoot. Is a nitrate fertilization, supplied either to the soil or IP T via foliar application, instrumental in decreasing toxic shoot concentrations of Cl−? There is also increasing indication for such Cl− antagonistic effects on the uptake and SC R translocation of phosphate [101,104] and sulphate [105]. A literature review reveals a dearth of published data on this topic and it is hardly known as to whether this antagonistic effect is either based on a specific competition for binding site at the phosphate or sulphate transport U protein or as to whether Cl− is simply leaking through these protein pores, quantitatively N displacing phosphate or sulphate. The latter would indicate an unspecific competition between A the Cl− and the anions. This and other open points, as for example the molecular identification M of the involved phosphate and sulphate transport proteins, await clarification. Work is also ED needed to clarify how this process can be better controlled to avoid macronutrient deficiency under Cl−-salinity. This topic is relevant because nitrogen, phosphate and sulphur are CC E nutrition. PT important ingredients in plant-based foods that determine the nutritional value for human 4. Dysfunctions under Cl−-toxicity and implication for food quality and safety A It is a little known fact that excess of Cl− in the environment interferes with the formation of quality of plant-based foods (and feeds) (Table 1). The interplay between Cl−-toxicities and quality-related aspects is reviewed in this chapter. 4.1 Protein biosynthesis 15 Ribosomal enzymes that catalyse protein synthesis are inhibited by high concentration of Cl −. Addition of 80 mM Cl− was inhibitory for the in vitro translation of RNA from the leaves of sugar beet (Beta vulgaris), wheat (Triticum aestivum), barley (Hordeum vulgare) or pea (Pisum sativum) [106]. This is one reason why plants need to avoid accumulation of Cl− in the cytoplasm when growing under Cl−-salinity [107]. Taking into account that synthesis of IP T ribosomes and proteins correlates closely with growth, it can be hypothesized that high cytoplasmic concentrations of Cl− restricts growth and development because a myriad of SC R enzymes and structural proteins may be affected in their abundance. It is worse testing as to whether the pattern of storage proteins of wheat (Triticum aestivum) changes under Cl−salinity. Wheat grain storage proteins such as glutenins and gliadins are important for food U quality for two reasons. First, they determine the backing quality of the dough [108] and, N second, they are cause of the food-related celiac disease [109]. Environmental stimuli such as A availability of nitrogen or sulphur have pronounced effects on the storage protein pattern M [110,111]. What about Cl−? It was very recently shown that Cl– fertilization on soils with low ED Cl– content can influence the protein concentration in the grain, which, however, is cultivarspecific [112]. A second link between supply of Cl– and protein synthesis was shown after PT treating maize with a stressful dose of Cl–: Abundances of 44 leaf proteins increased [113]. This effect was mediated via a Cl−-induced increase of the pH of the apoplast [17] that CC E transiently alkalinized in distant leaves upon the onset of Cl−-salinity [114]. Our understanding of why the apoplastic pH rises upon exposure to Cl−-stress is as rudimentary A [115], however, this Cl−-induced pH event increases abundances of more than 40 proteins. Among them are proteins that function in protein biosynthesis, sucrose catabolism or the phenylpropanoid pathway, which determines sensory quality of plant-based foods [116]. Experiments are anticipated to investigate the effect of Cl−-stress on backing quality and other quality determining features. 16 4.2 Blossom-end rot Blossom-end rot is a disorder that occurs on the fruits of tomato (Lycopersicon esculentum Mill.), eggplant (Solanum melongena), or pepper (Capsicum annuum). First, a water-soaked spot appears which may expand before it dries, flattens and darkens. Calcium deficiency is associated with the establishment of this a physiological disorder [117]. It is thought that IP T calcium demand in fast growing fruits exceeds its delivery, leading to a weakening of the cell wall and disturbed membrane integrity [118,119]. Studies on pepper [120] and tomato [46] SC R show that toxic concentrations of Cl− can initiated blossom-end rot. Here, a hypothesis for the induction of blossom-end in fruits is suggested under conditions of Cl−-salinity: It is known that Cl−, when given together with sodium, accumulates in the tissue where it weakly U associates to the oxygen of the lipid head-groups of the lipid membrane bilayers. In result, the N dipole moment that is normally created by the polarized water under non-saline conditions A switches, causing both, (i) alterations in the electrostatic potential and (ii) a thickening of the M membrane [121,122]. These salt-induced structural changes in the bilayer might induce ED problems during cell expansion, because, as a cell grows, its plasma membrane must expand but should not thicken. Since under Cl−-salinity, blossom-end rot is almost exclusively PT observed in the expanding tissues of the fruits, it is hypothesized that structural plasma CC E membrane impairments contribute to the formation of this disorder. 4.3 Ethylene production A It was reported for persimmon fruits (Dyospiros lotus L.) that Cl− stress affects the postharvest quality via effects exerted on ethylene production. Cl− accumulation in the fruit calyces (sepal) resulted in necrotic lesions and was correlated with increased ethylene production in the calyx. Calyx ethylene production stimulated autocatalytic ethylene production in fruit tissues diminishing fruit firmness by accelerating fruit maturity. As a 17 result, postharvest period is shortened due to reduced shelf life [123]. Knowledge with regard to the regulatory molecules downstream of Cl− and upstream of ethylene is lacking. Enhance cadmium uptake IP T 4.4 High Cl−-concentrations in nutrient solution were found to result in an enhanced accumulation SC R of cadmium in brown mustard (Brassica juncea; [124]). Similar results were reported for soils when chloro-complexation of cadmium increased the plant availability of cadmium [125,126]. This can be a problem for the quality of crops as shown for wheat. High inputs of Cl− increase U wheat grain cadmium levels above the dietary intake limits [127]. These examples shows that Loss of quality of fruit juices and beverages in response to Cl−-toxicity ED 4.5 M A increase fertility of soils that are rich in cadmium. N Cl− enriched fertilizers such as KCl, manures [91] or sewage sludge should not be used to Most grapevine varieties (Virti vinifera) are sensitive to Cl−-salinity because Cl− is loaded PT excessively into the root xylem and travels towards the shoot [74], where it accumulates and causes cellular damage [128]. Apparently, the Cl− is also streaming into the berries. This is a CC E little known fact. A screening of Australian grape juices revealed a mean concentration of Cl− in the juices of 140 mg/L [129]. The content of Cl− in grape juice will reflect that of the wine A [130] which is a negative because Cl− confers an unwanted salty taste. A few Australian viticultural regions contain such high soil Cl− concentrations that the mean concentration of Cl− in the juices were, according to the Australian food law, too high for selling the wine on the Australian market. In this case, wine makers may blend their product in order to sell it [129]. Not only the terroir but also the grape variety influences the concentration of Cl−- in the wine. The Syrah variety is prone to accumulate the highest amounts, giving the wine an 18 undesirable salty taste, which decreases its market appeal [131,132]. Besides salty taste, soapy attributes were correlated and associated with increasing Cl− concentrations in Chardonnay wines as it was found by a whole-mouth gustatory descriptive sensory evaluation conducted by a trained panel [133]. Cl− influx into the plants and particular into the fruits is also a problem for citrus (Citrus) IP T cultivation. Citrus production is particular limited by Cl−-salinity because oranges, grapefruits and lemons decrease fruit yield upon the accumulation of Cl− [134,135]. It was reported that SC R irrigation with Cl−-containing water reduced branch growth and increased Cl− levels in citrus leaves causing leaf damage [136]. Citrus also accumulates Cl− in fruit juices when irrigated with Cl−-containing waters [137], which is an unwanted product feature (salty taste). U Although there is improvement in our understanding of the molecular uptake mechanisms of N Cl− into the vegetative tissues of the root or leaf [10,11,34,72-74,138], there is lack of A information about the molecular mechanisms that facilitate transfer of Cl− into the fruits of our M crops. Which transport proteins are involved? What controls these proteins? Information ED about this may be useful for engineering trees that are able to exclude Cl− from being loaded 5. PT into the fruits. Cl− fertilization for fungal infection management CC E Crops may benefit from Cl− fertilization via a Cl−-induced disease repression [139]. Knowledge with regard to this is relevant as a fungal contamination of plant-based foods A constraints quality. The severity of take-all root rot (caused by Gaeumannomyces graminis var. tritici) has been decreased in winter wheat in response to the Cl− fertilization. This was discussed to be attributable to a Cl−-induced change in plant water potential as the growth of G. graminis tritici declines with declining water potential [140]. Such a general effect on water availability may also be the reason why foliar application of Cl−, sprayed as KCL, inhibits the spore germination of Septori tritici and Blumeria graminis f. sp. tritici on winter 19 wheat [141]. Beside foliar disease, a positive role of Cl− in supressing soil born diseases has also been shown for rhizoctonia disease in sugar beet [142]. More examples for the suppression of fungal disease by Cl−-containing salts are given elsewhere [143]. Based on this it can be assumed that a Cl− fertilization of infected plants may increases yields via the repression of fungal colonisations rather than being the direct result of an improved nutrition IP T with Cl−. There is a huge gap in our understanding about the molecular mechanisms that link Cl− fertilization with an enhanced tolerance towards fungal infections. The question raises as SC R to whether a Cl− foliar application modulates genetic networks that may convey a tolerance. A recent study demonstrated the link between supply of a stressful dose of Cl− and the Concluding remarks N 6. U accumulation of the plant defence-compounds glucosinolates [144]. A Cl− is a nutrient that is indispensable for photosynthesis and relevant for other processes such M as osmo- and turgor-regulation. Moreover, fertilization of Cl− can (i) improve fruit quality, as ED shown for strawberry [145], (ii) influence protein concentration in the grains of wheat [112], or (iii) may increases yields via the repression of fungal leaf infections [139-142]. PT Deficiencies, however, do not occur in most agricultural production systems because traces of Cl− in soil and rainwater are enough to fulfil requirement of nearly all major crops. In CC E contrast, excess of Cl− under conditions of Cl−-salinity induces severe physiological dysfunctions that hamper yield formation and affect quality-related attributes. For instance, A potato tuber development is impaired having low starch content, tomatoes suffer under Cl−induced nitrogen deficiency, grape juices and derived wines taste salty and post-harvestattributes such as shelf life of persimmon fruits is impaired by interactions between Cl−salinity and ethylene production (Figure 1). Surprisingly, the physiological and molecular reasons behind these toxicity symptoms are only poorly understood or, with respect to roots, almost not studied. Accumulation of excess of Cl− into the shoots might be avoided by 20 engineering crops that have either mechanism that facilitate Cl−-efflux from the roots [146,147] or that restrict xylem loading [9,148]. More research is necessary to identify the rate-limiting transport proteins in the diverse crop species and experiments are needed to understand how they are controlled. Improvements in knowledge should be utilized in breeding efforts to engineer crops that can grow in environments with high Cl−-concentrations IP T without showing impairments in quality or yield. When soils are only modestly contaminated with Cl−, it might be a strategy to fertilize mineral nitrate because this anion can supress the 7. SC R uptake of Cl− by means of antagonistic uptake competition. Outstanding questions Based on the finding that ribosomal enzymes are inhibited by high concentration of N U Cl−, it would be important to know as to whether the pattern of the seed storage A proteins is also affected. Wheat storage proteins such as glutenins and gliadins M determine the baking quality of the dough. Changes thereof are also relevant for the potential of wheat products to cause celiac disease. ED Fruit juices and derived wines can be enriched in Cl−, which confers an unwanted salty and soapy taste. The elucidation of the transporter proteins that facilitate uptake of Cl− PT from the vegetative tissues into the berry flesh would be helpful to engineer fruit crops that can exclude Cl− from the pulp. CC E Blossom-end rot is a disorder that frequently coincidences with Cl−-toxicity. How are toxic concentrations of Cl− mechanistically linked with these dark spots on the fruits? A Are membrane damages involved? 21 References [1] P. Stout, C. Johnson, T. Broyer, Chlorine in plant nutrition: Experiments with plants in nutrient solutions establish chlorine as a micronutrient essential to plant growth. Calif. Agric. 10(9) (1956) 10-10. [2] J.A. Raven, Chloride: essential micronutrient and multifunctional beneficial ion. J Exp [3] IP T Bot. erw421. 68(3) (2017) 359-367. G. Xu, H. Magen, J. Tarchitzky, U. Kafkafi, Advances in chloride nutrition of plants. [4] SC R Adv Agron. 68 (2000) 97-150. H. L. Bohn, B.L. McNeal, G.A. O'Connor, Soil chemistry, 3rd., Wiley, New York., 2001. O.K. Borggaard, Influence of iron oxides on the non-specific anion (chloride) U [5] W. De Vos, et al., Geochemical Atlas of Europe. Part 2. Interpretation of geochemical A [6] N adsorption by soil. J Soil Sci. 35(1) (1984) 71-78. [7] ED Finland, Espoo, 2006. M maps, additional tables, figures, maps and related publications. Geological Survey of J.L. Moya, A. Gómez-Cadenas, E. Primo-Millo, M. Talon, Chloride absorption in salt- PT sensitive Carrizo citrange and salt-tolerant Cleopatra mandarin citrus rootstocks is linked to water use. J Exp Bot. 54(383) (2003) 825-833. H. Gong, et al., Contrast in chloride exclusion between two grapevine genotypes and CC E [8] its variation in their hybrid progeny. J. Exp. Bot. 62(3) (2011) 989-999. A [9] B. Li, et al. Identification of a stelar-localised transport protein that facilitates root-toshoot transfer of chloride in Arabidopsis. Plant Physiol. 170(2) (2016) 1014-1029. [10] B. Li, M. Tester, M. Gilliham, Chloride on the Move. TIPS. 22(3) (2017) 236–248. [11] S. Wege, M. Gillihamand, S.W. Henderson, Chloride: not simply a ‘cheap osmoticum’, but a beneficial plant macronutrient. J. Exp. Bot. erx050 (2017). DOI: 10.1093/jxb/erx050 22 [12] H. Lessani, H. Marschner, Relation between salt tolerance and long-distance transport of sodium and chloride in various crop species. Funct Plant Biology. 5(1) (1978) 2737. [13] A. De Angeli, J. Zhang, S. Meyer, E. Martinoia, AtALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening in Arabidopsis. Nature [14] IP T Comm. 4 (2013) 1804. Y. Umena, K. Kawakami, J.R. Shen, N. Kamiya, Crystal structure of oxygen-evolving [15] SC R photosystem II at a resolution of 1.9 Å. Nature. 473(7345) (2011) 55-60. S. Schubert, Pflanzenernährung, Grundwissen Bachelor. Ulmer UTB, Stuttgart, Germany, 2006. H. Marschner, Marschner's mineral nutrition of higher plants. 3rd edition, Academic U [16] C.M. Geilfus, K.H. Mühling, Ratiometric monitoring of transient apoplastic A [17] N press, 2011. M alkalinizations in the leaf apoplast of living Vicia faba plants: chloride primes and ED PM–H+‐ATPase shapes NaCl‐induced systemic alkalinizations. New Phytol. 197(4) (2013) 1117-1129. C.M. Geilfus, K.H. Mühling, Microscopic and macroscopic monitoring of adaxial– PT [18] abaxial pH gradients in the leaf apoplast of Vicia faba L. as primed by NaCl stress at CC E the roots. Plant Sci. 223 (2014) 109-115. [19] C.M. Geilfus, et al., Fast responses of metabolites in Vicia faba L. to moderate NaCl A stress. Plant Physiol Biochem. 92 (2015) 19-29. [20] P.J. White, M.R. Broadley, Chloride in soils and its uptake and movement within the plant: a review. Ann Bot. 88(6) (2001) 967-988. [21] G.D. Schwenke, S.R. Simpfendorfer, B.C.Y. Collard, Confirmation of chloride deficiency as the cause of leaf spotting in durum wheat grown in the Australian northern grains region. Crop Pasture Sci. 66(2) (2015) 122-134. 23 [22] G.S. Smith, C.J. Clark, P.T. Holland, Chlorine requirement of kiwifruit (Actinidia deliciosa). New Phytol. 106(1) (1987) 71-80. [23] W. Bergmann, Ernährungsstörungen bei Kulturpflanzen: Entstehung, visuelle und analytische Diagnose/Hrsg.: Bergmann, Werner, 1983. T.C. Broyer, A.B. Carlton, C.M. Johnson, P.R. Stout, Chlorine - a micronutrient element for higher plants. Plant Physiol. 29(6) (1954)526. [25] IP T [24] J.T. Romo, M.R. Haferkamp, Effects of osmotic potential, potassium chloride, and SC R sodium chloride on germination of greasewood (Sarcobatus vermiculatus). Great Basin Nat. (1987) 110-116. [26] TJ. Flowers, Chloride as a nutrient and as an osmoticum. In: Tinker PB Laüchli A , J. Fromm, W. Eschrich, Correlation of ionic movements with phloem unloading and N [27] U eds. Advances in plant nutrition , Vol. 3. New York: Praeger, 1988, pp. 55–78. M.R.G. Roelfsema, R. Hedrich, In the light of stomatal opening: new insights into ‘the M [28] A loading in barley leaves. Plant Physiol Biochem. 27 (1989) 577-585. [29] ED Watergate’. New Phytol. 167(3) (2005) 665-691. R. Hedrich, Voltage-dependent chloride channels in plant cells: identification, PT characterization, and regulation of guard cell anion channel. Curr Topics Membr. 42 (1994) 1-33. A.M. Hetherington, Guard cell signaling. Cell. 107(6) (2001) 711-714. [31] K.I. Shimazaki, M. Doi, S.M. Assmann, T. Kinoshita, Light regulation of stomatal CC E [30] A movement. Annu Rev Plant Biol. 58 (2007) 219-247. [32] H. Kollist, M. Jossier, K. Laanemets, and S. Thomine, Anion channels in plant cells. FEBS J. 278(22) (2011) 4277-4292. [33] M. Escalante-Pérez, et al., Special pair of phytohormones controls excitability, slow closure, and external stomach formation in the Venus flytrap. Proc Natl Acad Sci U S A. 108(37) (2011) 15492-15497. 24 [34] M.R.G. Roelfsema, R. Hedrich, D. Geiger, Anion channels: master switches of stress responses. TIPS. 17(4) (2012) 221-229. [35] Z.M. Pei, J.M. Ward, J.F. Harper, J.I. Schroeder, A novel chloride channel in Vicia faba guard cell vacuoles activated by the serine/threonine kinase, CDPK. EMBO J. 15(23) (1996) 6564. K.A. Churchill, H. Sze, Anion-sensitive, H+-pumping ATPase of oat roots direct IP T [36] effects of Cl-, NO3-, and a disulfonic stilbene. Plant Physiol. 76(2) (1984) 490-497. S.K. Randall, H. Sze, Properties of the partially purified tonoplast H+-pumping SC R [37] ATPase from oat roots. J Biol Chem. 261(3) (1986) 1364-1371. [38] Y. Mathieu, et al., Regulation of vacuolar pH of plant cells I. Isolation and properties U of vacuoles suitable for 31P NMR studies. Plant Physiol. 89(1) (1989) 19-26. H.H. Felle, pH regulation in anoxic plants. Ann Bot. 96(4) (2005) 519-532. [40] R. Hedrich, et al., General mechanisms for solute transport across the tonoplast of A N [39] [41] ED 101(1) (1988) 7-13. M plant vacuoles: a patch‐clamp survey of ion channels and proton pumps. Plant Biol. R.F. Davis, Electrical properties of the plasmalemma and tonoplast in Valonia [42] PT ventricosa. Plant Physiol. 67(4) (1981) 825-831. L.B. Smart, F. Vojdani, M. Maeshima, T.A. Wilkins, Genes involved in CC E osmoregulation during turgor-driven cell expansion of developing cotton fibers are differentially regulated. Plant Physiol. 116(4), (1998) 1539-1549. A [43] [44] J.D. Franco-Navarro, et al., Chloride regulates leaf cell size and water relations in tobacco plants. J Exp Bot. 67(3) (2016) 873-891. Z.C. Chen, N. Yamaji, M. Fujii-Kashino, J.F. Ma, A cation-chloride cotransporter gene is required for cell elongation and osmoregulation in rice. Plant Physiol. 171(1) (2016) 494-507. 25 [45] A. Hager, M. Helmle, Properties of an ATP-fueled, Cl--dependent proton pump l ocalized in membranes of microsomal vesicles from maize coleoptiles. Z. Naturforsch. C. 36(11-12) (1981) 997-1008. [46] F.M. Eaton, Toxicity and accumulation of chloride and sulfate salts in plants. J Agric Res. 64(7) (1942) 357-399. E. Barbez, K. Dünser, A. Gaidora, T. Lendl, W. Busch, Auxin steers root cell IP T [47] expansion via apoplastic pH regulation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. [48] SC R U.S.A. 114(24) (2017) E4884-E4893. O. Babourina, S. Shabala, I.A.N. Newman, Auxin stimulates Cl− uptake by oat coleoptiles. Ann. Bot. 82(3) (1998) 331-336. R. Lamond, V. Martin, C. Olsen, K. Rector, Chloride fertilization increases yields of U [49] N.W. Christensen, R.G. Taylor, T.L. Jackson, B.L. Mitchell, Chloride effects on water A [50] N corn and grain sorghum. Better Crops 84(4) (2000) 10-11. [50] ED 73(6) (1981) 1053-1058. M potentials and yield of winter wheat infected with take-all root rot. Agronomy Journal. H. Sze, J.M. Ward, S. Lai, Vacuolar H+-translocating ATPases from plants: structure, [51] PT function, and isoforms. J Bioenerg Biomembr. 24(4) (1992) 371-381. H. Sze, X. Li, M.G. Palmgren, Energization of plant cell membranes by H+-pumping CC E ATPases: regulation and biosynthesis. Plant Cell. 11(4) (1999) 677-689. [52] J. Dettmer, A. Hong-Hermesdorf, Y.D. Stierhof, K. Schumacher, Vacuolar H+-ATPase A activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell. [53] 18(3) (2006) 715-730. M. Schrempf, R.L. Satter, A.W. Galston, Potassium-linked chloride fluxes during rhythmic leaf movement of Albizzia julibrissin. Plant Physiol. 58(2) (1976) 190-192. [54] M. Pietruszka, J. Stolarek, K. Pazurkiewicz-Kocot, Time evolution of the action potential in plant cells. J Biol Phys. 23(4) (1997) 219-232. 26 [55] E. Davies, Action potentials as multifunctional signals in plants: a unifying hypothesis to explain apparently disparate wound responses. P, C & E. 10(8) (1987) 623-631. [56] S. Scherzer, et al., The Dionaea muscipula ammonium channel DmAMT1 provides NH4+ uptake associated with Venus flytrap’s prey digestion. Curr Biol. 23(17) (2013) 1649-1657. S.E. Rognes, Anion regulation of lupin asparagine synthetase: chloride activation of IP T [57] the glutamine-utilizing reactions. Phytochem. 19(11) (1980) 2287-2293. K.J. Na, H.J. Lee, Role of chloride ion as an allosteric activator of angiotensin- SC R [58] converting enzyme. Arch Biochem Bioph 227(2) (1983)580-586. [59] R. Pokhrel, I.L. McConnell, G.W. Brudvig, Chloride regulation of enzyme turnover: U application to the role of chloride in photosystem II. Biochemistry. 50(14) (2011) A N. Aghajari, G. Feller, C. Gerday, R. Haser, Structural basis of α‐amylase activation M [60] N 2725-2734. by chloride. Protein Sci. 11(6) (2002) 1435-1441. H. Haehn, H. Schweigart, Zur Kenntnis der Kartoffelamylase. Biochem. Z. 143 (1923) ED [61] 516-526. O. Warburg, W. Lüttgens, Fotokhimicheskoye vostan- ovlenye khinona v zelenykh PT [62] granulakh [Photochemical reduction of quinone in green granules.] Biokhim. 11 CC E (1946) 301-322. [63] D.I. Arnon, F.R. Whatley, Is chloride a coenzyme of photosynthesis? Science. A 110(2865) (1949) 554-556. [64] P.R. Gorham, K.A. Clendenning, Anionic stimulation of the Hill reaction in isolated chloroplasts. Arch Biochem Biophys. 37(1) (1952) 199-223. [65] N. Terry, Photosynthesis, growth, and the role of chloride. Plant Physiol. 60(1) (1977) 69-75. 27 [66] P.H. Homann, The relation between the chloride status of the photosynthetic water splitting complex and the inhibitory effectiveness of amines. Photosynth Res. 10(3) (1986) 497-505. [67] H. Greenway, R. Munns, Mechanisms of salt tolerance in nonhalophytes. Ann Rev Plant Physiol. 31(1) (1980) 149-190. B. Hanson, S.R. Grattan, A. Fulton, Agricultural salinity and drainage. University of California Irrigation Program, University of California, Davis, 1999. G. Niu, D.S. Rodriguez, Responses of growth and ion uptake of four rose rootstocks to SC R [69] IP T [68] chloride-or sulfate-dominated salinity. J Am Soc Hortic Sci. 133(5) (2008) 663-669. [70] S.P. Robinson, W.J.S. Downton, Potassium, sodium, and chloride content of isolated U intact chloroplasts in relation to ionic compartmentation in leaves. Arch Biochem G. Schröppel-Meier, W.M. Kaiser, Ion homeostasis in chloroplasts under salinity and A [71] N Biophys. 228(1) (1984) 197-206. M mineral deficiency. Plant Physiol. 87(4) (1988) 828-832. S.D. Tyerman, Anion channels in plants. Annu Rev Plant Biol. 43(1) (1992) 351-373. [73] N.L. Teakle, S.D. Tyerman, Mechanisms of Cl‐transport contributing to salt ED [72] S.W. Henderson, et al., Chloride exclusion and salt tolerance in grapevine is associated CC E [74] PT tolerance. P, C & E. 33(4) (2010) 566-589. with differential ion transporter expression in roots. BMC Plant Biol. 14(1) (2014) 273. A [75] D.H. Sanchez, et al., Comparative ionomics and metabolomics in extremophile and glycophytic Lotus species under salt stress challenge the metabolic pre‐adaptation hypothesis. P. C. & E. 34(4) (2011) 605-617. 28 [76] J. Brumos, M. Talon, R.Y.M. Bouhlaland, J.M. Colmenero-Flores, Cl‐homeostasis in includer and excluder citrus rootstocks: transport mechanisms and identification of candidate genes. P, C & E. 33(12) (2010) 2012-2027. [77] T.J. Flowers, T.D. Colmer, Salinity tolerance in halophytes. New Phytol. 179(4) (2008) 945-963. J. Rozema, et al., Comparing salt tolerance of beet cultivars and their halophytic IP T [78] ancestor: consequences of domestication and breeding programmes. AoB Plants. 7 [79] SC R (2015) plu083. C. Critchley, Stimulation of photosynthetic electron transport in a salt-tolerant plant by J. Bose, R. Munns, S. Shabala, M. Gilliham, B. Pogson, S.D. Tyerman, Chloroplast N [80] U high chloride concentrations. Nature, 298(5873) (1982) 483-485. A function and ion regulation in plants growing on saline soils: lessons from halophytes. [81] M J. Exp. Bot. 68(12) (2017) 3129-3143. Y.P. Dang, et al., High subsoil chloride concentrations reduce soil water extraction and ED crop yield on Vertosols in north-eastern Australia. Crop Pasture Sci. 59(4) (2008) 321330. H.L. Bohn, D.G. Strawn, G.A. O'Connor, Soil chemistry. John Wiley & Sons, New PT [82] York., 2015. C. Slabu, C. Zörb, D. Steffens, S. Schubert, Is salt stress of faba bean (Vicia faba) CC E [83] caused by Na+ or Cl– toxicity? J Plant Nutr Soil Sci. 172(5) (2009) 644-651. A [84] E. Tavakkoli, P. Rengasamy, G.K. McDonald, High concentrations of Na+ and Cl− ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J Exp Bot. 61(15) (2010) 4449-4459. [85] U. Heber, H.W. Heldt, The chloroplast envelope: structure, function, and role in leaf metabolism. Ann Rev Plant Physiol. 32(1) (1981) 139-168. 29 [86] J.R. Seemann, C. Critchley, Effects of salt stress on the growth, ion content, stomatal behaviour and photosynthetic capacity of a salt-sensitive species, Phaseolus vulgaris L. Planta, 164(2) (1985) 151-162. [87] E. Hason-Porath, A. Poljakoff-Mayber, Effect of chloride and sulphate types of salinity on the nicotinamide-adenine-dinucleotides in pea root tips. J Exp Bot. 21(2) [88] IP T (1970) 300-303. Y. Bar, U. Kafkafi, E. Lahav, Nitrate nutrition as a tool to reduce chloride toxicity in [89] SC R avocado. Yearbook, South African Avocado Growers Association. 10 (1987) 47-48. L., Duan, et al., Endodermal ABA signaling promotes lateral root quiescence during salt stress in Arabidopsis seedlings. Plant Cell. 25(1) (2013) 324-341. C.D. Van Loon, W. Van den Berg, The effect of chloride fertilization on blackspot U [90] N susceptibility and other quality characteristics and on yield of potato. Potato Res. 46(3- R. Moral, et al., Salinity, organic content, micronutrients and heavy metals in pig M [91] A 4) (2003) 147-154. [92] ED slurries from South-eastern Spain. Waste Manag. 28(2) (2008) 367-371. H. Zhong, The influence of chlorine on the physiology of potato. Acta Agriculturae [93] PT Zhejiangensis. 5(2) (1993) 83-88. S. S. Baslavskaja, Influence of the chloride ion on the content of carbohydrates in CC E potato leaves. Plant Physiol. 11(4) (1936) 863. [94] H. Beringer, K. Koch, M.G Lindhauer, Source: sink relationships in potato (Solanum A tuberosum) as influenced by potassium chloride or potassium sulphate nutrition. Plant [95] Soil. 124(2) (1990) 287-290. M. Reich, T. Aghajanzadeh, J. Helm, S. Parmar, M.J. Hawkesford, L.J. De Kok, Chloride and sulfate salinity differently affect biomass, mineral nutrient composition and expression of sulfate transport and assimilation genes in Brassica rapa. Plant Soil. 411(1-2) (2017) 319-332. 30 [96] M. Cerezo, P. Garcı́a-Agustı́n, M.D. Serna, E. Primo-Millo, Kinetics of nitrate uptake by Citrus seedlings and inhibitory effects of salinity. Plant Sci. 126(1) (1997) 105-112. [97] S.H. Lin, et al., Mutation of the Arabidopsis NRT1. 5 nitrate transporter causes defective root-to-shoot nitrate transport. Plant Cell. 20(9) (2008) 2514-2528. [98] C.Z. Chen, X.F. Lv, J.Y. Li, H.Y. Yi, J.M. Gong, Arabidopsis NRT1. 5 is another IP T essential component in the regulation of nitrate reallocation and stress tolerance. Plant Physiol. 159(4) (2012) 1582-1590. Y. Hu, U. Schmidhalter, Spatial distributions and net deposition rates of mineral SC R [99] elements in the elongating wheat (Triticum aestivum L.) leaf under saline soil conditions. Planta. 204(2) (1998) 212-219. U [100] N. Abbaspour, B. Kaiser, S. Tyerman, Root apoplastic transport and water relations N cannot account for differences in Cl− transport and Cl−/NO3− interactions of two A grapevine rootstocks differing in salt tolerance. Acta Physiol Plant. 36(3) (2014) 687- M 698. ED [101] I. Papadopoulos, V.V. Rendig, Interactive effects of salinity and nitrogen on growth and yield of tomato plants. Plant Soil. 73(1) (1983) 47-57. PT [102] B.W. Hütsch, W. He, S. Schubert, Nitrogen nutritional status of young maize plants (Zea mays) is not limited by NaCl stress. J Plant Nutr Soil Sci. 179(6) (2016) 775-783. CC E [103] U. Kafkafi, N. Valoras, J. Letey, Chloride interaction with nitrate and phosphate nutrition in tomato (Lycopersicon esculentum L.). J Plant Nutr. 5(12) (1982) 1369- A 1385. [104] D. Massa, N.S. Mattson, H.J Lieth, Effects of saline root environment (NaCl) on nitrate and potassium uptake kinetics for rose plants: a Michaelis–Menten modelling approach. Plant Soil. 318 (1-2) (2009) 101-115. [105] R.P. Mor, H.R. Manchanda, Influence of phosphorus on the tolerance of table pea to chloride and sulfate salinity in a sandy soil. Arid Land Res Manag. 6(1) (1992) 41-52. 31 [106] T.S. Gibson, J. Speirs, C.J. Brady, Salt‐tolerance in plants. II. In vitro translation of m‐RNAs from salt‐tolerant and salt‐sensitive plants on wheat germ ribosomes. Responses to ions and compatible organic solutes. P, C & E. 7(8) (1984) 579-587. [107] T.J. Flowers, R. Munns, T.D. Colmer, Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann Bot. 115(3) (2014) 419-431. IP T [108] H. Wieser, S. Antes, W. Seilmeier, Quantitative determination of gluten protein types in wheat flour by reversed-phase high-performance liquid chromatography. Cereal SC R Chem. 75(5) (1998) 644-650. [109] H. Wieser, P. Koehler, The biochemical basis of celiac disease. Cereal Chem. 85(1) (2008) 1-13. N U [110] C. Zörb, et al., Quantitative protein composition and baking quality of winter wheat as A affected by late sulfur fertilization. J Agr Food Chem. 57(9) (2009) 3877-3885. M [111] C. Zörb, C. Grover, D. Steinfurth, K.H. Mühling, Quantitative proteome analysis of wheat gluten as influenced by N and S nutrition. Plant Soil. 327(1-2) (2010) 225-234. ED [112] C. Graham, H. Woodard, A. Bly, P. Fixen, R. Gelderman, Chloride fertilizers increase spring wheat yields in the northern great plains. Agronomy Journal, 109(1) (2017) PT 327-334. [113] C.M. Geilfus, R. Tenhaken, S.C. Carpentier, Transient alkalinization of the leaf CC E apoplast stiffens the cell wall during onset of chloride-salinity in corn leaves J. Biol. Chem. 2017. DOI: jbc.M117.799866. doi:10.1074/jbc.M117.799866. A [114] C.M. Geilfus, K.H. Mühling, H. Kaiser, C. Plieth, Bacterially produced Pt-GFP as ratiometric dual-excitation sensor for in planta mapping of leaf apoplastic pH in intact Avena sativa and Vicia faba. Plant Methods. 10(1) (2014) 31. [115] C.M. Geilfus, The pH of the apoplast – dynamic factor with functional impact under stress. Mol. Plant. 10(11) (2017) 1371–1386. 32 [116] A. Kumar, B.E. Ellis, The phenylalanine ammonia-lyase gene family in raspberry. Structure, expression, and evolution. Plant Physiol. 127(1) (2001) 230-239. [117] M.D. Taylor, S.J. Locascio, Blossom-end rot: a calcium deficiency. J Plant Nutr. 27(1) (2004) 123-139. or a stress-related disorder? Sci Hort. 90(3) (2001) 193-208. IP T [118] M.C. Saure, Blossom-end rot of tomato (Lycopersicon esculentum Mill.)—a calcium- [119] L.C. Ho, P.J. White, A cellular hypothesis for the induction of blossom-end rot in SC R tomato fruit. Ann Bot. 95(4) (2005) 571-581. [120] J.M. Navarro, C. Garrido, M. Carvajal, V. Martinez, Yield and fruit quality of pepper plants under sulphate and chloride salinity. J Hortic Sci Biotech. 77(1) (2002) 52-57. O. Edholm, J.J. Perez, Effect of ions U [121] A. Cordomi, on a dipalmitoyl N phosphatidylcholine bilayer. A molecular dynamics simulation study. J Phys Chem B. A 112(5) (2008) 1397-1408. M [122] B. Klasczyk, V. Knecht, R. Lipowsky, R. Dimova, Interactions of alkali metal ED chlorides with phosphatidylcholine vesicles. Langmuir. 26(24) (2010) 18951-18958. [123] C. Besada, et al., Chloride stress triggers maturation and negatively affects the PT postharvest quality of persimmon fruit. Involvement of calyx ethylene production. Plant Physiol Biochem. 100 (2016) 105-112. CC E [124] U.J. López‐Chuken, S.D. Young, J.L. Guzmán‐Mar, Evaluating a ‘biotic ligand model’applied to chloride‐enhanced Cd uptake by Brassica juncea from nutrient A solution at constant Cd2+ activity. Environ Tech. 31(3) (2010) 307-318. [125] E. Smolders, M.J. McLaughlin, Chloride increases cadmium uptake in Swiss chard in a resin-buffered nutrient solution. Soil Sci Soc Am J. 60(5) (1996) 1443-1447. [126] K. Weggler, M.J. McLaughlin, R.D. Graham, Effect of chloride in soil solution on the plant availability of biosolid-borne cadmium. J Environ Qual. 33(2) (2004) 496-504. 33 [127] A.S. Dahlin, J. Eriksson, C.D. Campbel, I. Öborn, Soil amendment affects Cd uptake by wheat—are we underestimating the risks from chloride inputs? Sci Total Environ. 554 (2016) 349-357. [128] C. Sybesma, (Ed.)., Advances in photosynthesis research: Proceedings of the VIth international congress on photosynthesis, Brussels, Belgium, August 1–6, 1983 (Vol. IP T 2). Springer, 2013. [129] P.A. Leske, A.N. Sas, A.D. Coulter, C.S., Stockley, T.H. Lee, The composition of SC R Australian grape juice: chloride, sodium and sulfate ions. Aust J Grape Wine Res. 3(1) (1997) 26-30. [130] B.C. Rankine, J.C.M. Fornachon, E.W. Boehm, K.M. Cellier, Influence of grape U variety, climate and soil on grape composition and on the composition and quality of N table wines. Vitis. 10 (1971) 33-50. A [131] M.S. Coli, A.G.P. Rangel, E.S. Souza, M.F. Oliveira, A.C.N. Chiaradia, Chloride M concentration in red wines: influence of terroir and grape type. Food Sci Technol ED (Campinas). 35(1) (2015) 95-99. [132] R.R. Walker, et al.,. Effects of the rootstock Ramsey (Vitis champini) on ion and PT organic acid composition of grapes and wine, and on wine spectral characteristics. Aust J Grape Wine Res. 4(3) (1998) 100-110. CC E [133] L.C. de Loryn, et al., Evaluation of sensory thresholds and perception of sodium chloride in grape juice and wine. J Enol Vitic. ajev-2013. (2013) A [134] E.V. Maas, Salinity and citriculture. Tree Physiol. 12(2) (1993) 195-216. [135] J. Shalhevet, D. Yaron, U. Horowitz, Salinity and citrus yield — an analysis of results from a salinity survey. J Hortic Sci. 49(1) (1974) 15-27. [136] Y. Bar, A. Apelbaum, U. Kafkaf, R. Goren, Polyamines in chloride-stressed Citrus plants: alleviation of stress by nitrate supplementation via irrigation water. J Am Soc Hortic Sci. 121(3) (1996) 507-513. 34 [137] J.P. Syvertsen, M.L. Smith, B.J. Boman, Tree growth, mineral nutrition and nutrient leaching losses from soil of salinized citrus. Agric Ecosyst Environ. 45(3) (1993) 319334. [138] H. Barbier-Brygoo, et al., Anion channels in higher plants: functional characterization, molecular structure and physiological role. Biochim Biophys Acta. 1465(1) (2000) IP T 199-218. [139] J.R. Heckman, Chlorine. In: Barker, A.T., Pilbeam, D. (Eds.), Handbook of Plant SC R Nutrition. CRC Press/Taylor & Francis Group, Boca Raton, FL, pp. 279e291. Cook, J.W., 1997. The effect of foliar applied fertilisers on leaf diseases of cereals. PhD thesis, Harper Adams University College, Newport, UK (2006) 274 pp. U [140] Y.S. Kwak, D.M. Weller, Take-all of wheat and natural disease suppression: a review. N Plant Pathol. J. 29(2) (2013) 125-135. A [141] J.W. Cook, The effect of foliar applied fertilisers on leaf diseases of cereals. PhD M thesis, Harper Adams University College, Newport, UK (1997) 274 pp. ED [142] W.H. Elmer, Influence of chloride and nitrogen form on Rhizoctonia root and crown rot of table beets. Plant Dis. 81(6) (1997) 635-640. PT [143] T. Deliopoulos, P.S. Kettlewell, M.C. Hare, Fungal disease suppression by inorganic salts: A review. Crop Prot. 29(10) (2010) 1059-1075. CC E [144] T.A. Aghajanzadeh, M. Reich, S. Kopriva, L.J. De Kok, Impact of chloride (NaCl, KCl) and sulphate (Na2SO4, K2SO4) salinity on glucosinolate metabolism in Brassica A rapa. J. Agron. Crop Sci. (2017) DOI: 10.1111/jac.12243 [145] S. Bellof, S. Schubert, Chloride improves fruit yield and quality of strawberry (Fragaria× ananassa Duch.). In The Proceedings of the International Plant Nutrition Colloquium XVI. (2009). 35 [146] J. Sun, et al., NaCl-induced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species. Plant Physiol. 149(2) (2009) 11411153. [147] B. Li, et al., AtNPF2. 5 modulates chloride (Cl−) efflux from roots of Arabidopsis thaliana. Front Plant Sci. 7:2013 (2016). doi: 10.3389/fpls.2016.02013. IP T [148] N.L. Teakle, T.J. Flowers, D. Realand, T.D. Colmer, Lotus tenuis tolerates the interactive effects of salinity and waterlogging by ‘excluding’ Na+ and Cl− from the SC R xylem. J. Exp. Bot. 58(8) (2007) 2169-2180. [149] N. Bernstein, M. Ioffe, M. Zilberstaine, Salt-stress effects on avocado rootstock growth. I. Establishing criteria for determination of shoot growth sensitivity to the U stress. Plant Soil. 233(1) (2001) 1-11. N [150] F.T. Bingham, L.B. Fenn, J.J. Oertli, A sandculture study of chloride toxicity to A mature avocado trees. Soil Sci Soc Am J. 32(2) (1968) 249-252. M [151] P.J. Cole, Chloride toxicity in citrus. Irrigation Sci. 6(1) (1985) 63-71. ED [152] M. Prasad, G.K. Burge, T.M. Spiers, G. Fietje, Chloride‐induced leaf breakdown in kiwifruit. J. Plant Nutri. 16(6) (1993) 999-1012. PT [153] V.D. Zuazo, A.M. Raya, J.A. Ruiz, Impact of salinity on the fruit yield of mango (Mangifera indica L. cv.‘Osteen’). Eur. J. Agron. 21(3) (2004) 323-334. CC E [154] V.D. Zuazo, A.M. Raya, J.A. Ruiz, D.F. Tarifa, Impact of salinity on macro-and micronutrients uptake in mango (Mangifera indica L. cv. Osteen) with different A rootstocks. Span. J. Agric. Res. 2(1) (2004) 121-133. [155] F. Visconti, D.S. Intrigliolo, A. Quiñones, L. Tudela, L. Bonet, J.M. de Paz, Differences in specific chloride toxicity to Diospyros kaki cv.“Rojo Brillante” grafted on D. lotus and D. virginiana. Sci. Hortic. 214 (2017) 83-90. [156] M.B. Parker, G.J. Gascho, T.P. Gaines, Chloride toxicity of soybeans grown on Atlantic coast flatwoods soils. Agron. J. 75(3) (1983) 439-443. 36 [157] Y. Mizrahi, D. Pasternak, Effect of salinity on quality of various agricultural crops. Plant Soil, 89(1) (1985) 301-307. [158] M. Esna-Ashari, M. Gholami, The effect of increased chloride (Cl-) content in nutrient solution on yield and quality of strawberry (Fragaria ananassa Duch.) fruits. J. Fruit Ornam. Plant Res. 18(1) (2010) 37-44. IP T [159] H. Ishizaki, T. Akiya, Effects of chlorine on growth and quality of tobacco. Japan A CC E PT ED M A N U SC R Agric. Res. Q. 12 (1978) 1–6. 37 Figure legend IP T Figure 1. Cl−-salinity induces physiological dysfunctions in many crops constraining both yield and quality formation. Potatoes produce smaller tubers with lower starch content, grape SC R juices and derived wines taste salty and post-harvest-attributes such as shelf life of persimmon fruits is impaired by interactions between Cl−-salinity and autocatalytic ethylene production. U Figure file A CC E PT ED M A N Figure 1 38 I N U SC R Table 1. Physiological dysfunctions under Cl−-toxicity and implication for food quality and yield Crop Symptom and implication for food quality Reference [46] Medicago sativa Avocado tree Persea americana Shoot growth depression Dampened root growth impairs water supply, reduced fruit production possibly due to dehydration, leaf necrosis and premature leaf abscission Barley Hordeum vulgare Burned leaf tips, yield loss, potentially inhibitory for translation of RNA [46,81,106] Canola Brassica spp Yield loss [81] Chickpea Cicer arietinum Yield loss M A Alfalfa [88,149,150] [81] − Citrus spp. Yield loss, reduced leaf and branch growth, leaf yellowing, leaf bronzing, burned tips, leaf abscission, accumulation of Cl in fruit juices [46,135,137,151] Common bean Phaseolus vulgaris Leaf burning and abscission [46] Corn Zea may Cotton ED Citrus [46] Gossypium hirsutum Shoot growth depression [46] Field bean Vicia faba [83,84] Grapevine Vitis vinifera Shoot growth depression, chlorophyll degradation, reduction in photosynthetic capacity and quantum yield Growth reduction, lack of nitrogen in the shoot, accumulation of Cl− in berries which confers salty taste Kiwifruit Actinida deliciosa Leaf scorch, leaf drop, reduction of phosphor and nitrogen leaf content [22,152] Mango Mangifera indica L. Chloride accumulates in fruits, defoliation due to chloride burn [153,154] Pisum sativum Growth reduction, lack of nitrogen in the shoot, depression of mitochondrial respiration in root [87,105,106] Pepper Capsicum annuum Decreased fruit yield and fruit size, blossom-end rot results in fewer marketable fruits [120] Persimmon fruits Dyospiros lotus Necrotic lesions, ethylene production in the calyx reduces fruit firmness and shelf life [123,155] Potato Solanum tuberosum Decreased tuber yield, retarded shoot growth and emergence, decline in photosynthesis, impaired nitrogen uptake [90,92,93] Rose Rosa odorata Shoot growth reduction and detracted aesthetic appearance [69] Sorghum Sorghum vulgare Leaf burning and leaf-edge necrosis [46] Soybean Glycine max Leaf scorch and yield reduction [103,156] Strawberry Fragaria ananassa [158] Tomato Lycopersicon esculentum Increased total fruit yield and fruit firmness. Restricted growth and impaired fruit setting, lack of shoot nitrogen, increased defoliation, blossom-end rot, reduced fruit water content, putative positive aspect on aromatic compounds in fruits. Tobacco Nicotiana tabacum Light green leaves and leaf margins curled upwards, lowering of burning quality, ash shows black colour, aroma and taste aggravate. [159] Wheat Triticum aestivum Yield loss, lack of nitrogen in the shoot, potentially inhibitory for translation of RNA [81,90,106] Wheat Triticum durum Yield loss [81] CC E A Pea PT Burned leaf tips and growth reduction [74,100,131] [1,3,46,101,157] 39 This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. A CC E PT ED M A N U SC R IP T Conflicts of interest: none 40