Proteins, Amino Acids, and Peptide Bonds Presentation
advertisement

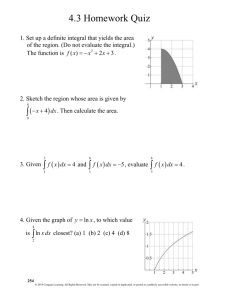
Proteins © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 1 Proteins (1 of 2) Proteins serve many functions, including the following: • 1. Structure: Collagen and keratin are the chief constituents of skin, bone, hair, and nails. • 2. Catalysis: Virtually all reactions in living systems are catalyzed by proteins called enzymes. • 3. Movement: Muscles are made up of proteins called myosin and actin. • 4. Transport: Hemoglobin transports oxygen from the lungs to cells, other proteins transport molecules across cell membranes. • 5. Hormones: Many hormones are proteins, among them insulin, oxytocin, and human growth hormone. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 2 Proteins (2 of 2) • 6. Protection: Blood clotting involves the protein fibrinogen; the body used proteins called antibodies to fight disease. • 7. Storage: Casein in milk and ovalbumin in eggs store nutrients for newborn infants and birds. Ferritin, a protein in the liver, stores iron. • 8. Regulation: Certain proteins not only control the expression of genes, but also control when gene expression takes place. • Proteins are divided into two types: • Fibrous proteins • Globular proteins © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 3 Amino Acids Amino acid: A compound that contains both an amino group and a carboxyl group. • α-Amino acid: An amino acid in which the amino group is on the carbon adjacent to the carboxyl group. • Although α-amino acids are commonly written in the un-ionized form, they are more properly written in the zwitterion (internal salt) form. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 4 Chirality of α-Amino Acids (1 of 2) With the exception of glycine, all protein-derived amino acids have at least one stereocenter (the α-carbon) and are chiral. • The vast majority of α-amino acids have the L-configuration at the α-carbon. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 5 Chirality of α-Amino Acids (2 of 2) Figure 21.2 Alanine and glyceraldehyde stereochemistry © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 6 Protein-Derived α-Amino Acids (1 of 4) Nonpolar side chains. Each ionizable group is shown in the form present in highest concentration at pH 7.0). © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 7 Protein-Derived α-Amino Acids (2 of 4) • Polar side chains (at pH 7.0) © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 8 Protein-Derived α-Amino Acids (3 of 4) Acidic and basic side chains (at pH 7.0) © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 9 Protein-Derived α-Amino Acids (4 of 4) 1. For 19 of the 20, the α-amino group is primary; for proline, it is secondary. 2. With the exception of glycine, the α-carbon of each is a stereocenter. 3. Isoleucine (left) and threonine (right) contain a second stereocenter. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 10 Ionization vs. pH (1 of 2) The net charge on an amino acid depends on the pH of the solution in which it is dissolved. • If we dissolve an amino acid in water, it is present in the aqueous solution as its zwitterion. • If we add a strong acid such as HCl to bring the pH of the solution to pH 0, the strong acid donates a proton to the –COO– of the zwitterion turning it into a positive ion. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 11 Ionization vs. pH (2 of 2) • If we add a strong base such as NaOH to the solution and bring its pH to 14, a proton is transferred from the NH3+ group to the base turning the zwitterion into a negative ion. • To summarize: © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 12 Isoelectric Point (pI) • Isoelectric point, pI: A pH at which a sample of amino acids or protein has an equal number of positive and negative charges. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 13 Peptides (1 of 2) Proteins are long chains of amino acids joined by amide bonds. • Peptide bond (peptide linkage): The special name given to the amide bond between the α-carboxyl group of one amino acid and the α-amino group of another. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 14 Peptides (2 of 2) • Peptide: A short polymer of amino acids joined by peptide bonds; they are classified by the number of amino acids in the chain. • Dipeptide: A molecule containing two amino acids joined by a peptide bond. • Tripeptide: A molecule containing three amino acids joined by peptide bonds. • Polypeptide: A macromolecule containing many amino acids joined by peptide bonds. • Protein: A biological macromolecule containing at least 30 to 50 amino acids joined by peptide bonds. • The individual amino acid units are often referred to as “residues.” © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 15 Writing Peptides By convention, peptides are written from the left to right, beginning with the free –NH3+ group and ending with the free –COO– group. • C-terminal amino acid: The amino acid at the end of the chain having the free –COO– group. • N-terminal amino acid: The amino acid at the end of the chain having the free –NH3+ group. • Alternatively they are referred to as the C-terminus and the N-terminus. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 16 Phe, Trp, and Tyr The amino acids phenylalanine, tryptophan, and tyrosine have aromatic rings on their side chains. Tryptophan is the precursor to the neurotransmitter serotonin. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 17 Tyr and Phe Phenylalanine and tyrosine are precursors to norepinephrine and epinephrine, both of which are stimulatory neurotransmitters. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 18 Other Amino Acids Figure 21.3 Hydroxylation (oxidation) of proline, lysine, and tyrosine, respectively and iodination for tyrosine, give these uncommon amino acids. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 19 Protein Properties (1 of 2) Figure 21.4 A small peptide showing the direction of the peptide chain (N-terminal to C-terminal). © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 20 Peptide Bond • Figure 21.5. A peptide bond is typically written as a carbonyl group bonded to an N–H group. Linus Pauling, however, discovered that there is about 40% double bond character to the C–N bond and that a peptide bond between two amino acids is planar, which Pauling explained using the concept of resonance. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 21 Protein Properties (2 of 2) Proteins behave as zwitterions. Proteins have an isoelectric point, pI. • At its isoelectric point, the protein has no net charge. • At any pH above (more basic than) its pI, it has a net negative charge. • At any pH below (more acidic than) its pI, it has a net positive charge. • Hemoglobin, for example, has an almost equal number of acidic and basic side chains; its pI is 6.8. • Serum albumin has more acidic side chains; its pI is 4.9. • Proteins are least soluble in water at their isoelectric points and can be precipitated from solution when pH = pI. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 22 Levels of Structure • Primary structure: The sequence of amino acids in a polypeptide chain. Read from the N-terminal amino acid to the C-terminal amino acid. • Secondary structure: Conformations of amino acids in localized regions of a polypeptide chain. Examples are α-helix, β-pleated sheet, and random coil. • Tertiary structure: The complete three-dimensional arrangement of atoms of a polypeptide chain. • Quaternary structure: The spatial relationship and interactions between subunits in a protein that has more than one polypeptide chain. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 23 Primary Structure (1 of 5) • Primary structure: The sequence of amino acids in a polypeptide chain. • The number peptides possible from the 20 protein-derived amino acids is enormous. • There are 20 × 20 = 400 dipeptides possible. • There are 20 × 20 × 20 = 8000 tripetides possible. • The number of peptides possible for a chain of n amino acids is 20n. • For a small protein of 60 amino acids, the number of proteins possible is 2060 ≅ 1078, which is possibly greater than the number of atoms in the universe! © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 24 Primary Structure (2 of 5) Figure 21.7 The hormone insulin consists of two polypeptide chains, A and B, held together by two disulfide bonds. The sequence shown here is for bovine insulin. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 25 Primary Structure (3 of 5) How important is the exact amino acid sequence? • Human insulin consists of two polypeptide chains having a total of 51 amino acids; the two chains are connected by two interchain disulfide bonds. • In Table 21.2 are differences between four types of insulin. A Chain positions 8-9-10 B Chain position 30 Human -Thr-Ser-Ile -Thr Cow -Ala-Ser-Val- -Ala Hog -Thr-Ser-Ile- -Ala Sheep -Ala-Gly-Val- -Ala © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 26 Primary Structure (4 of 5) • Vasopressin and oxytocin are both nonapeptides but have quite different biological functions. • Vasopressin is an antidiuretic hormone. • Oxytocin affects contractions of the uterus in childbirth and the muscles of the breast that aid in the secretion of milk. • Figure 21.8 The structures of vasopressin and oxytocin. Differences are shown in color. (next screen) © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 27 Primary Structure (5 of 5) © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 28 Secondary Structure Secondary structure: Conformations of amino acids in localized regions of a polypeptide chain. • The most common types of secondary structure are α-helix and β-pleated sheet. • α-Helix: A type of secondary structure in which a section of polypeptide chain coils into a spiral, most commonly a right-handed spiral. • β-Pleated sheet: A type of secondary structure in which two polypeptide chains or sections of the same polypeptide chain align parallel to each other; the chains may be parallel or antiparallel. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 29 Secondary Structure: The α-Helix Figure 21.9(a) The α-Helix. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 30 α-Helix In a section of α-helix • The six atoms of each peptide bond lie in the same plane. • The N–H groups of peptide bonds point in the same direction, roughly parallel to the axis of the helix. • The C=O groups of peptide bonds point in the opposite direction, also roughly parallel to the axis of the helix. • The C=O group of each peptide bond is hydrogen bonded to the N–H group of the peptide bond four amino acid units away from it. • All R– groups point outward from the helix. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 31 β-Pleated Sheet (1 of 2) Figure 21.9(b) The β-pleated sheet structure. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 32 β-Pleated Sheet (2 of 2) In a section of β-pleated sheet; • The six atoms of each peptide bond of a β-pleated sheet lie in the same plane. • The C=O and N–H groups of the peptide bonds from adjacent chains point toward each other and are in the same plane so that hydrogen bonding is possible between them. • All R– groups on any one chain alternate, first above, then below the plane of the sheet, etc. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 33 Random Coil Figure 21.10 A random coil. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 34 Secondary Structure (1 of 2) • Many globular proteins contain all three kinds of secondary structure in different parts of their molecules: α-helix, β-pleated sheet, and random coil (next screen). © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 35 Secondary Structure (2 of 2) Figure 21.11 Schematic structure of the enzyme carboxypeptidase. The β-pleated sheet sections are shown in blue, the α-helix portions in green, and the random coils as orange strings. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 36 The Collagen Triple Helix Figure 21.12 The collagen triple helix. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 37 Tertiary Structure (1 of 2) Tertiary structure: the overall conformation of an entire polypeptide chain. Tertiary structure is stabilized in four ways: • • • • Covalent bonds as for example, the formation of disulfide bonds between cysteine side chains. Hydrogen bonding between polar groups of side chains, as for example between the –OH groups of serine and threonine. Salt bridges, as for example, the attraction of the –NH3+ group of lysine and the –COO– group of aspartic acid. Hydrophobic interactions, as for example, between the nonpolar side chains of phenylalanine and isoleucine. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 38 Cysteine The –SH (sulfhydryl) group of cysteine is easily oxidized to an –S–S– (disulfide). © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 39 Tertiary Structure (2 of 2) Figure 21.14 Forces that stabilize tertiary structures of proteins. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 40 Quaternary Structure (1 of 4) Quaternary structure: The arrangement of polypeptide chains into a noncovalently bonded aggregation. • The individual chains are held together by hydrogen bonds, salt bridges, and hydrophobic interactions. Hemoglobin • Adult hemoglobin: Two alpha chains of 141 amino acids each, and two beta chains of 146 amino acids each. • Fetal hemoglobin: Two alpha chains and two gamma chains. Fetal hemoglobin has a greater affinity for oxygen than does adult hemoglobin. • Each chain surrounds an iron-containing heme unit. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 41 Quaternary Structure (2 of 4) Figure 21.20 The quaternary structure of hemoglobin. The structure of heme is shown on the next screen. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 42 Quaternary Structure (3 of 4) The structure of heme. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 43 Quaternary Structure (4 of 4) Integral membrane proteins form quaternary structures in which the outer surface is largely nonpolar (hydrophobic) and interacts with the lipid bilayer. Two of these are shown on the next screens. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 44 Denaturation (1 of 2) Denaturation: The process of destroying the native conformation of a protein by chemical or physical means. • Some denaturations are reversible, while others permanently damage the protein. Denaturing agents include: • Heat: Heat can disrupt hydrogen bonding; in globular proteins, it can cause unfolding of polypeptide chains with the result that coagulation and precipitation may take place. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 45 Denaturation (2 of 2) • 6 M aqueous urea: Disrupts hydrogen bonding. • Surface-active agents: Detergents such as sodium dodecylbenzenesulfate (SDS) disrupt hydrogen bonding. • Reducing agents: 2-Mercaptoethanol (HOCH2CH2SH) cleaves disulfide bonds by reducing –S–S– groups to –SH groups. • Heavy metal ions: Transition metal ions such as Pb2+, Hg2+, and Cd2+ form water-insoluble salts with –SH groups; Hg2+ for example forms –S–Hg–S–. • Alcohols: 70% ethanol penetrates bacteria and kills them by coagulating their proteins. It is used to sterilize skin before injections. © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 46 The End © 2020 Cengage. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 47