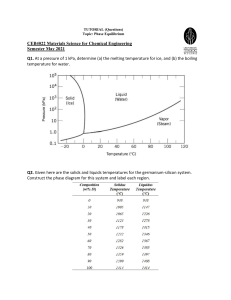
EGN 3365 Review for Exam #2 1. A 0.001 in. BCC iron foil is used to separate a high hydrogen gas from a low hydrogen gas at 650C. 5x108 atoms/cm3 are in equilibrium on one side on one side of the foil, and 2x103 H atoms/cm3 are in equilibrium with the other side. Additional diffusion info from handbook: H in BCC Iron, Qd is 3,600 cal/mol, Do is 0.0012 cm2/s, R is 1.987 cal/ mol-K Determine: (a) The concentration gradient of hydrogen, (b) The flux of hydrogen through the foil. 2. A 0.80% C steel must operate at 950C in an oxidizing environment where the carbon content at the steel surface is zero. Only the outer most 0.2 cm of the steel part can fall below 0.75% C. What is the maximum time the steel part can operate? (Steel is C in FCC iron.) 3. What temperature is required to obtain 0.50 %C at a distance 0.5 mm beneath the surface of a 0.20 %C steel in 2 hours, when 1.10 %C is present at the surface? Assume that the iron is FCC. 1. Is it possible to have a copper‐silver alloy that, at equilibrium , consists of β phase of composition 92 %wt Ag – 8 wt% Cu, and also a liquid phase of composition 76 wt% Ag –24wt% Cu? If so, what will be the approximate temperature of the alloy? If this is not possible, explain why. 2. A 50 wt% Ni – 50wt% Cu alloy is slowly cooled form 1400°C to 1200°C. a. At what temperature does the first solid phase form? b. What is the composition of this solid phase? c. At what temperature does the liquid solidify? d. What is the composition of the last remaining liquid phase? e. 3. A 65 wt% Ni – 35wt% Cu alloy is heated to a temperature within the α+liquid phase region. If the composition of the α phase is 70 wt% Ni, determine a. The temperature of the alloy b. The composition of the liquid phase c. The mass fractions of both phases. 4. The microstructure of a copper‐silver alloy at 775°C consists of primary α and eutectic structures. If the mass fractions of these two microconstituents are 0.73 and 0.27, respectively, determine the composition of the alloy. 5. Consider 2.5 kg of austenite containing 0.65wt% C¸ cooled below 727°C a. What is the proeutectiod phase? b. How many kilograms of each of total ferrite and cementite form? c. How many kilograms each of pearlite and the proeutectoid phase form? d. Schematically sketch and label the resulting microstructure. 6. Compute the mass fraction of eutectoid cementite in an iron‐carbon alloy that contains 1.00 wt % C.