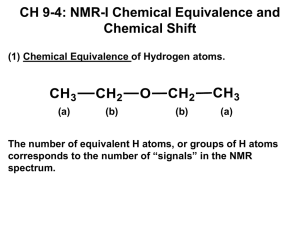
NUCLEAR MAGNETIC RESONANCE (NMR) SPECTROSCOPY INTRODUCTION Nuclear magnetic resonance spectroscopy(NMR) • is a sensitive, non-destructive method for elucidating the structure of organic molecules. • is a powerful analytical technique used to characterize organic molecules by identifying carbon-hydrogen frameworks within molecules. • is a research technique that exploits the magnetic properties of certain atomic nuclei. NMR is the most powerful tool for organic structure determination. • used to study a wide variety of nuclei • EMR used in NMR have long wavelength (10 7 to 108 μ) • And extremely low energy • These radiations are able to interact with the nuclei of certain atoms which are exposed to a strong magnetic field Chapter 13 3 Nuclear Spin : the total angular momentum of a nucleus denoted by Mass Num. Odd Even Even Atomic Num. Odd or Even Even Odd Spin Quantum Num. Example 1H, 13C, 19F ½, 3/2, 5/2… etc 12C, 16O, 32S 0 1, 2, 3… The number of possible orientations = 2I + 1. So in case of 1H, (I = 1/2), possible orientation will be two. Chapter 13 4 • Two common types of NMR spectroscopy are used to characterize organic structure: • 1H NMR:- Used to determine the type and number of H atoms in a molecule • 13C NMR:- Used to determine the type of carbon atoms in the molecule. Principle of NMR • The Principle behind NMR comes from the spin of a nucleus and it generates a magnetic field. Without an external applied magnetic field, the nuclear spin are random in directions. But when an external magnetic field is present the nuclei align themselves either with or against the field of the external magnet. • If an external magnetic field is applied, an energy transfer (ΔE) is possible between ground state to excited state. • When the spin returns to its ground state level, the absorbed radiofrequency energy is emitted at the same frequency level. • The emitted radiofrequency signal that gives the NMR of the concerned nucleus. Principle of NMR • The emitted radiofrequency is directly proportional to the strength of the applied field, ꝨBₒ Ʋ= 2𝜋 Where, Bₒ = External magnetic field experienced by proton Ꝩ = Magnetogyric ration (the ratio between the nucear magnetic moment and angular moment) The nucleus of each hydrogen atom behaves like a tiny magnet, which usually lines up with an applied magnetic field. If a molecule containing hydrogen is placed in a strong magnetic field, the hydrogen nucleus can line up with the field or line up against it! N S N S N S N S Nucleus spin aligned with the field – Low energy! Nucleus spin aligned against the field – High energy! A photon (from radio frequency region) with the right amount of energy can be absorbed and cause the spinning proton to flip. The NMR Spectrometer Instrumentation The NMR spectrophotometer consists of following components; Magnet:- A strong magnet provides stable and homogenous field. The magnet size is 15 inches in diameter and capable of producing strong fields up to 23,500 gauss for 100MHz. Sample and sample holder:- A 1 – 30 mg sample is used in the form of dilute solution (2 – 10%) and solvent doesn’t contain hydrogen of its own ions. The sample holder is glass tube about 5mm in diameter and 15 – 20 cm in length. Radiofrequency oscillator:- The RF oscillator is installed perpendicular to magnetic field and transmits radio waves of some mixed frequency such as 60, 100, 220, 300 MHz. a sweep generator is installed to supply dc current to sec. magnet. RF detector or Receiver:- It is installed perpendicular to both magnetic field and the oscillator coil and is tuned to the same frequency as transmitter. When precession frequency is match with RF the nuclei induces (emf) in detector coil and this signal is amplified and sent to recorder. Recorder :- The recorder gives a spectrum as a plot of strength resonance signal on Y axis & strength of magnetic field on X axis. The strength of resonance signal is directly proportional to number of nuclei resonating at that particular field strength. Working of Instrumentation • In NMR spectrophotometer, the sample is dissolved in Deuterated Solvent and placed in long cylindrical glass tube especially made for NMR and also add small amount of TMS as internal reference. • Placed the sample tube in gap between two magnetic poles where coil is attached to a specific RF generator (e.g. 60 MHz). This coil supply EMR energy required to change spin orientation of proton. • Then radiofrequency generator irradiates the sample with short pulse of radiation causing resonance. As magnetic field strength increase, the precessional frequency of all protons increase and when this frequency proton reaches 60 MHz, the resonance occurs. • As magnetic field increase linearly the recorder pen travels from left to right, thus protons which achieve resonance faster i.e. (Deshielded) appears on left side (downfield), where as those protons (Shielded) appears on right side (upfield) of chart in the form of peaks. • The superconducting magnet in modern NMR spectrometers have coils that are cooled in liquid helium and conduct electricity with no resistance. The NMR Graph NMR graph is characterized by • The number of the signals and their splitting • The location of the signals(chemical shift) • The intensity of the signal Chapter 13 16 The number of signals shows how many different kinds of protons are present. Splitting tells us about the neighboring protons. The intensity of the signal shows the number of protons of that type. The location of the signals shows how shielded or deshielded the proton is. Internal Standard: Tetramethylsilane (TMS) • TMS (Tetra methyl silane) is most commonly used as Internal standard for measuring the position of 1H, 13C and 29Si in NMR spectroscopy. Due to following reasons; ➢It is chemically inert and miscible with a large range of solvents. ➢Its twelve protons are all magnetically equivalent. ➢Its protons are highly shielded and gives a strong peak even in small quantity. CH3 CH3 Si CH3 CH3 Tetrameth yls ilane (TMS) ➢Si is less electronegative than carbon hence TMS protons are highly shielded. ➢It is highly volatile and can be easily removed to get back sample. ➢It does not take part in intermolecular associations with sample. ➢Organic protons absorb downfield (to the left) of the TMS signal. TMS is shown at a peak value of δ = 0 ppm. Solvents Used • The solvent used for dissolving sample should have following properties; ➢ Should not contain proton, ➢ Inexpensive ➢ Low boiling point and non polar in nature. • Generally deuterated chloroform CDCl3 is used as solvent. • If sample is soluble in polar solvent, then deuterium oxide (D 2O), DMSO, CCl4, CS2, CF3, COOH are used as solvent. Number of signals • Equivalent and non equivalent protons. • Each set of magnetically equivalent protons will give rise to a NMR signal. • Number of signals in NMR = number of different sets of equivalent protons • Magnetically equivalent protons are chemically equivalent Examples: CH4 all 4 hydrogens are identical i.e. in same environment hence ONE signal CH3COCH3 ONE signal CH3CH2Cl ethyl chloride CH3OH methanol CH3CH2CH3 propane H CH3 C H C CH3 isobutylene TWO signals • CH3CH2CH2Cl • CH3CH2NH2 THREE signals • CH3CH(OH)CH3 ClHC CH2 Trans to Cl H H C Cis to Cl H C Cl Splitting of signals • The splitting of the signal is related to the number of protons in neighboring groups. • Let us illustrate it by taking the example of ethyl bromide a CH3 b CH2 Br Two signals will be obtained. Splitting = (n + 1) rule N = number of neighboring protons Number of Neighboring protons 0 Splitting of peaks (n + l) rule 1 Termed as Intensity distribution Singlet 1 1 2 doublet 1:1 2 3 triplet 1:2:1 3 4 quartet 1:3:3:1 4 5 Pentate (multiplet) 1:4:6:4:1 Chemical Shift (Position of Signals) • The utility of NMR is that all protons do not show resonance at same frequency, because, it is surrounded by slightly different electronic environment from one another. Thus they absorb at different field strength. • When molecule placed in magnetic field, so its surrounding electron circulate & generates counter field which opposes the applied magnetic field on proton. If the resulting field feels by proton is high and that proton called as the Shielded proton. and if resulting field is low then that proton called as the Shielded proton. • Shielded proton shifts absorption signal to right side (upfield) and deshielded proton shifts absorption signal to left side (down field) of spectrum. • Such shifting in position of NMR absorption signals which arise due to the shielding or deshielding of proton by surrounding electrons are called as Chemical shift. • Position of signals in spectrum help us to know nature of protons i.e. aromatic, aliphatic, acetylinic, vinylic, adjacent to electron releasing or withdrawing grp. Chemical Shift Chemical shift, ppm = Shift downfield from TMS (Hz) Spectrometer frequency (MHz) • Measured in parts per million. • Ratio of shift downfield from TMS (Hz) to total spectrometer frequency (MHz). • Same value for 60, 100, or 300 MHz machine. • Called the delta scale. Factors affecting Chemical Shift • Shielding and Deshielding effects • Electronegativity CH3F CH3Cl CH3Br CH3I TMS Shielded proton Higher Field (up field) lower value of δ Deshielded proton Lower Field (down field) Higher value of δ Shielding or Deshielding Protons Shielding or Deshielding Protons Shielding or Deshielding Protons Chemical Shift (Position of Signals) Chemical shift depends upon following parameters: 2. Hybridization of adjacent atoms 1. Electro negativity of nearby atoms CH3 -X Electron egativity of X Chemical Shif t () CH3 F CH3 OH CH3 Cl CH3 Br CH3 I 4.0 3.5 3.1 2.8 2.5 4.26 3.47 3.05 2.68 2.16 ( CH3 ) 4 C ( CH3 ) 4 Si 2.1 1.8 0.86 0.00 Type of Hydrogen (R = alkyl) N ame of Hydrogen Chemical Sh ift () RCH3 , R2 CH2 , R3 CH Alk yl 0.8 - 1.7 R2 C=C(R)CHR2 Allylic 1.6 - 2.6 RC CH Acetylen ic 2.0 - 3.0 R2 C=CHR, R2 C=CH2 Vin ylic 4.6 - 5.7 RCHO Ald ehydic 9.5-10.1 3. Diamagnetic effects from adjacent pi bonds Type of H RCH3 RC CH R2 C=CH2 N ame Alk yl Acetylenic Vin ylic Chemical Shift () 0.8- 1.0 2.0 - 3.0 4.6 - 5.7 Delta Scale Chapter 13 34 Location of Signals • More electronegative atoms deshield more and give larger shift values. • Effect decreases with distance. • Additional electronegative atoms cause increase in chemical shift. Predict the number of signals and multiplicity of respective signals in the following: CH3CH3 CH2CH3 ClCH2CH2CH2Cl Cl2CHCH2Cl A compound has molecular formulae C10H14. It gives following NMR data: 0.88 δ (9 H, singlet) Assign the structure. 7.28 δ (5 H, singlet, ar. protons) NMR spectra of Ethane NMR spectra of Propane NMR spectra of Butane