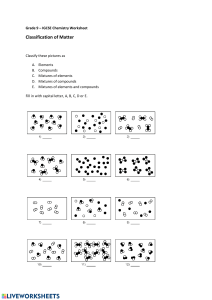
Name Date Pd Honors Chemistry – Unit 4 Worksheet 1 1. Identify the separation techniques pictured below. Which technique would be useful to separate a mixture of sand and salt? Of salt and water? 2. Explain why the technique at left would not be effective in separating a mixture of salt and sugar. 3. Draw particle representations for the following: A mixture of iron and sulfur A compound of iron and sulfur 4. Explain why a magnet can separate iron atoms from the mixture but not from the compound. Modeling Chemistry 1 U4 ws 1 5. Consider the four containers below. a. Which of these are mixtures? pure substances? b. Which contain only compounds? only elements 6. Consider the four containers below. a. Which of these are mixtures? pure substances? b. Which contain only compounds? only elements 7. Consider the four containers below. a. Which of these are mixtures? pure substances? b. Which contain only compounds? only elements 8. Which of the containers in #7 contain a gas? Modeling Chemistry 2 a liquid a solid U4 ws 1 9. Suppose you had a sample of water in which an unknown solid substance has been dissolved. Describe a procedure that you would use to effectively separate the substance from the water where you would recover both the substance AND the water. 10. Use complete sentences to discuss the difference between filtration, evaporation, and distillation. 11. Differentiate between elements and compounds. Illustrate the difference with a particle diagram. 12. Differentiate between compounds and mixtures. Use the terms "fixed composition" and "variable composition" in your response. 13. How do heterogeneous mixtures differ from homogeneous mixtures? Explain why homogeneous mixtures and solutions are synonymous. Modeling Chemistry 3 U4 ws 1 14. There is no such thing as a heterogeneous mixture made entirely of gases. Using what you know about gases and their behavior, propose an explanation for this fact. 6. Identify the following particle diagrams in terms of solid, liquid, gas, atom, molecule, element, compound, mixture, pure substance, homogeneous, or heterogeneous. List all that apply for each image. a. b. Modeling Chemistry 4 U4 ws 1