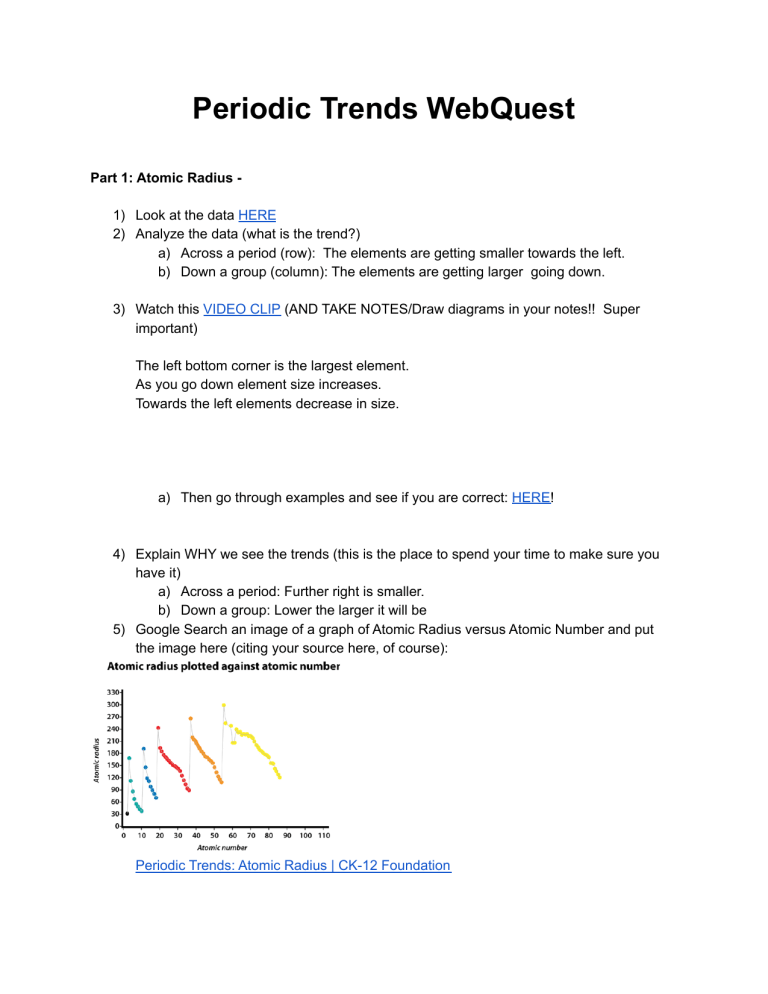
Periodic Trends WebQuest Part 1: Atomic Radius 1) Look at the data HERE 2) Analyze the data (what is the trend?) a) Across a period (row): The elements are getting smaller towards the left. b) Down a group (column): The elements are getting larger going down. 3) Watch this VIDEO CLIP (AND TAKE NOTES/Draw diagrams in your notes!! Super important) The left bottom corner is the largest element. As you go down element size increases. Towards the left elements decrease in size. a) Then go through examples and see if you are correct: HERE! 4) Explain WHY we see the trends (this is the place to spend your time to make sure you have it) a) Across a period: Further right is smaller. b) Down a group: Lower the larger it will be 5) Google Search an image of a graph of Atomic Radius versus Atomic Number and put the image here (citing your source here, of course): Periodic Trends: Atomic Radius | CK-12 Foundation 6) Does the data presented in the graph you found support your findings above (the trends across a period and down a group)? Why or why not? Are there any inconsistencies? Yes, it shows how the lower an atom is the larger the atomic radius is. 7) Time to practice: a) Which has a bigger atomic size AND explain WHY: Ca or Br? Br is bigger because it is further to the right. b) Which has a smaller atomic size AND explain WHY: Ca or Be? Be because it is higher up. Part 2: Ionization Energy - the energy required to rip a valence electron OFF of the atom (as in “the energy required to make an ion”) 8) Do some research first to make sure you understand the definition above: a) What does HIGH Ionization energy mean? The closer it is to the nucleus b) What does LOW ionization energy mean? The further the valence ring the lower it is. 9) Look at the data HERE 10) Analyze the data and the graph given in the link (what is the trend?) a) Across a period: Higher ionazation energy further to the right. b) Down a group: Further down higher ionazation energy. 11) Watch this VIDEO CLIP (AND TAKE NOTES/Draw diagrams in your notes!! Super important) 12) Explain WHY we see the trends (this is the place to spend your time to make sure you have it) a) Across a period: Towards the right their is more valance shells so less ionazation. b) Down a group: Down the group their is more valance shells so less ionazation. 13) Does the data presented in the graph in the link I gave you support your findings above (the trends across a period and down a group)? Why or why not? Are there any inconsistencies? Yes, because it shows the higher the atomic level the less ionazation nnergy. 14) Time to practice: a) Which has a higher ionization energy AND explain WHY: Ca or Br? Ca is higher because the valance shell is closer to the nucleus. b) Which has a lower ionization energy AND explain WHY: Ca or Be? Ca because the valance shell is further away from the nucleus. Part 3: Electronegativity - the attractiveness between an atom and outside electrons (basically, how much does the atom attract NEW electrons TOWARD it?) 15) Do some research first to make sure you understand the definition above: a) What does HIGH electronegativity mean? It is far from the nucleus b) What does LOW electronegativity energy mean? It is close to the nucleus 16) Look at the data HERE a) What is the range of numbers for electronegativity? What is the lowest and highest? Fluorine is assigned a value of 4.0, and values range down to cesium and francium which are the least electronegative at 0.7 17) Analyze the data and the graph given in the link (what is the trend?) a) Across a period: Lower b) Down a group: Lower 18) Watch this VIDEO CLIP (AND TAKE NOTES/Draw diagrams in your notes!! Super important) 19) Explain WHY we see the trends (this is the place to spend your time to make sure you have it) a) Across a period: The further away from the nucleus the higher the electronegativity. b) Down a group: The further away from the nucleus the higher the electronegativity. 20) Does the data presented in the graph in the link I gave you support your findings above (the trends across a period and down a group)? Why or why not? Are there any inconsistencies? No, it seems to be an abrupt shift in the data sometimes. 21) Time to practice: a) Which has a higher ionization energy AND explain WHY: Ca or Br? Ca is higher because the valance shell is closer to the nucleus. b) Which has a lower ionization energy AND explain WHY: Ca or Be? Ca because the valance shell is further away from the nucleus. Part 4: Challenge questions when you finish! 1. Now you are ready! Rank the following elements from smallest atomic size to largest atomic size: Na, O, Ba, Si O, Si, Na, Ba 2. How are Ionization energy and electronegativity easy to predict if you understand the atomic size trend? You can just look at the periodic table and understand how big it is.

