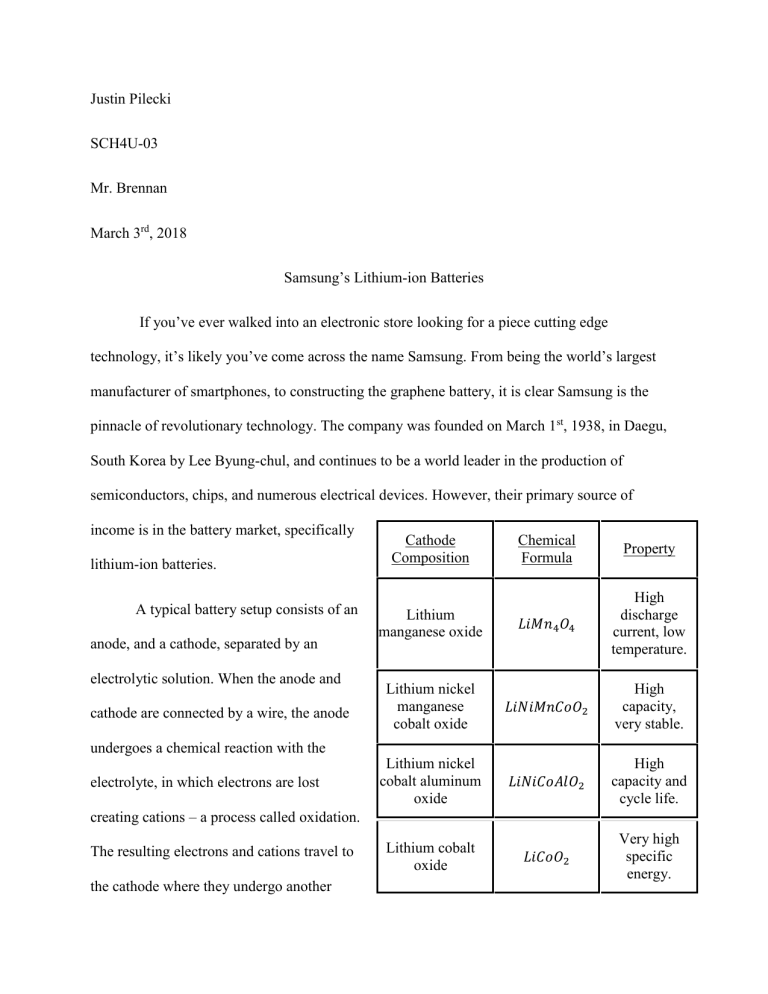
Justin Pilecki SCH4U-03 Mr. Brennan March 3rd, 2018 Samsung’s Lithium-ion Batteries If you’ve ever walked into an electronic store looking for a piece cutting edge technology, it’s likely you’ve come across the name Samsung. From being the world’s largest manufacturer of smartphones, to constructing the graphene battery, it is clear Samsung is the pinnacle of revolutionary technology. The company was founded on March 1st, 1938, in Daegu, South Korea by Lee Byung-chul, and continues to be a world leader in the production of semiconductors, chips, and numerous electrical devices. However, their primary source of income is in the battery market, specifically lithium-ion batteries. A typical battery setup consists of an anode, and a cathode, separated by an electrolytic solution. When the anode and cathode are connected by a wire, the anode Cathode Composition Chemical Formula Property Lithium manganese oxide 𝐿𝑖𝑀𝑛4 𝑂4 High discharge current, low temperature. Lithium nickel manganese cobalt oxide 𝐿𝑖𝑁𝑖𝑀𝑛𝐶𝑜𝑂2 High capacity, very stable. Lithium nickel cobalt aluminum oxide 𝐿𝑖𝑁𝑖𝐶𝑜𝐴𝑙𝑂2 High capacity and cycle life. Lithium cobalt oxide 𝐿𝑖𝐶𝑜𝑂2 Very high specific energy. undergoes a chemical reaction with the electrolyte, in which electrons are lost creating cations – a process called oxidation. The resulting electrons and cations travel to the cathode where they undergo another chemical reaction called reduction. In a lithium ion battery, the electrolyte is a salt solution containing lithium ions (hence the name), typically paired with a carbon or silicon based anode on one end, and a cathode at the other. The anode is nearly identical in composition throughout Samsung’s lithium-ion batteries, but a different choice of cathodic materials are used for specific purposes (the table above shows a list of cathodes and their properties). When the battery is placed in a device, the positively charged lithium ions are attracted to the cathode and intercalate (the process of inserting ions into a host lattice), where the electric current generated is used to power different items. As the cathode becomes negatively charged, the ions migrate towards it. Eventually, the reactants are used up however, but possess the ability to recharge. By plugging the battery into a socket, and pumping it with an electrical source, the anodic and cathodic reactions reverse back to their original states. As electric current is pushed through, the anode attracts the ions back into their original state, ready for another use. To get a glimpse of the chemistry behind the lithium-ion battery, the following chemical equation models a typical Samsung 18650 𝐿𝑖𝐶𝑜𝑂2 cathode and 𝐶6 𝐿𝑖 anode, and the reaction taking place: Cathode: 𝐿𝑖𝐶𝑜𝑂2 ⇆ 𝐶𝑜𝑂2 + 𝐿𝑖 + + 𝑒 − Anode: 𝐶6 + 𝐿𝑖 + + 𝑒 − ⇆ 𝐶6 𝐿𝑖 Note: The arrows pointing right represent the reactions as the battery charges, and the arrows pointing left, the discharging phase. Overall: LiCoO2 + 𝐶6 ⇆ 𝐶𝑜𝑂2 + 𝐶6 𝐿𝑖 As clearly shown, oxidation occurs at the anode where an electron is lost, and a lithium ion is formed. The electron travels to the cathode where it undergoes a reduction reaction, and forms lithium metal. It is in this reaction that generates the electricity to power different items. Samsung’s lithium-ion batteries are used for a variety of devices such as cell phones, laptops, and generally anything that is going to be used more than once. Lithium is king of the reducing agents, and thus offers incredibly high voltage per cell in each battery, putting it miles ahead of any other anode-cathode pair. Higher voltage means more energy per gram, so when placed side by side with any other battery of equal cell number, the lithium-ion will be able to output more energy as required. Other benefits include the fact that this battery does not have to be primed, which is advantageous to consumers who do not have any prior battery knowledge. Once out of the box, the lithium-ion battery’s capacity, is nearly one hundred percent usable, and does not require repeated charging and discharging to be at its peak performance. A nickel-based battery for example, has a certain specified capacity that in order to achieve, must be formatted through repeated discharge and charging cycles. Not only is this inconvenient, but even potentially dangerous for consumers who lack knowledge and misinterpret the process. Even though a great number of benefits have already been said towards the lithium-ion battery, it is only the tip of the iceberg as the battery also boasts extremely light weight (lithium is the lightest metal with an atomic weight of 6.94u), and superior cycle life. There are some drawbacks to this technology however, mainly in the environmental area and the cost. Lithium in general is a fairly abundant metal, but the materials to make the traditional cathode (cobalt based) are not. Cobalt is quite a rare metal and difficult to obtain. Combining it with the fact that this is a new technology in the development phase, paired with rare ingredients, makes the price reach lengths of up to fifty percent greater, compared to other batteries. The actual manufacturing process is not so eco-friendly either, as production of nickel and cobalt cathodes releases toxic substances into the environment. Humans as well as animals can suffer from adverse respiratory, pulmonary, and neurological side effects after prolonged exposure. Resource depletion is another factor to consider when constructing these batteries, as continued mining could result in a shortage of these precious metals. Products like stainless steel (nickel is used for this) and jet engine alloys (cobalt based), would be harder to come by, and will likely increase in price. Unfortunately, there is not much ways to combat these side effects except recycling the batteries, which is entirely up to the consumer or to use different cathodic materials. Building eco-friendly factories is just not plausible at this moment, so there will always be some environmental emission. Despite producing amazing technology, and being a multi-billion dollar company, Samsung actually treats its workforce quite poorly, particularly the lower class. There has been numerous complaints of extremely long work hours, ranging up to sixteen hours per day with one rest day per month. And, according to local labor laws after an audit, Samsung was reported to work workers, three to six times the legal overtime limit. Even more suspicious behavior ensued, when in 2013, the company stated that all future audits would be conducted by an independent third party. Child laborers were also said to be spotted in one of Samsung’s suppliers, but there was no conclusive evidence. Overall, this is quite a bad company to work for if one is not in the upper-class of the workforce, i.e. a software engineer or product manager, but Samsung does provide numerous benefits, free food, and transport for these upper-class individuals. This is definitely detrimental to the company, as an interdependent balance between the upper and lower class must be established to maximize profit, product quality, and customer satisfaction. Even though the physical conditions of the workplace are satisfactory, new rules must be implemented to achieve equilibrium between the classes.



