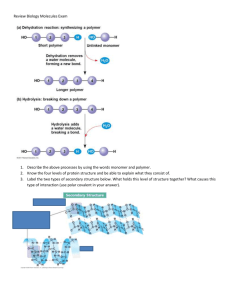
Biology Exam Cheat Sheet WaterPhysical properties of water are explained by hydrogen bonding between the individual molecules The polarity of water makes it a good polar solvent One water molecule may form hydrogen bonds with up to four other water molecules Water has a high specific heat capacity is very high-The amount of heat required to raise 1kg of water through 1 degree Note- The combination of cohesion, adhesion and surface tension work to create the capillary action in plants COHESION AND ADEHESION Water molecules are capable of forming hydrogen bonds with other polar substances Cohesion- Attraction between water molecules Adhesion-The attraction between water molecule and other polar substance Surface tension allows small animals to walk on water - The property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of its molecules Water is a solvent Polar molecules have dipoles that are attracted to the dipoles in the water Hydrophilic is used to refer to substances that readily dissolve in water Water as a transport medium- Cohesion allows water to move in the same direction without breaking apart The flow of water is used to transport other substances which are able to dissolve in the universal sol Nutrients, oxygen, proteins and waste products What can’t water dissolve? Density and freezing point Ice floats on water because it is less dense than water Water is most dense at 4 degrees. At temperatures below 4 degrees, the density of water decreases Specific Heat Capacity- This refers to the energy needed to raise the temperature of 1kg of a substance by 1 degree. Since water has a high specific heat capacity, a lot of heat energy is required to raise its temperature. Due to hydrogen bonding. Heat that is added to the water is not used to raise the temperature of the water but rather to break the bonds. Therefore, the sea on a summer day will not increase drastically and sea life can survive The body temperature remains relatively stable during large changes in temperature Latent heat of vaporization- A measure of the energy required to transform a given quantity of a substance from a liquid to a gas Large amounts of energy are required to break the hydrogen bonds between molecules of liquid water. This allows for watery environments to maintain stable conditions Sweating has a cooling effect. The water absorbs heat energy and evaporates, leaving the skin cooler Latent heat of fusion- Water at 0 degrees needs to lose a lot of heat energy in order for it to form ice crystals. Thus, cell contents and aquatic environments are slow to freeze in cold weather Transparency- Water is transparent to visible light Allows aquatic plants to obtain light for photosynthesis Water participates in many chemical reactions in metabolism Water is difficult to compress Water has a high tensile strength; means that water columns do not break or pull apart easily. Continuous columns of water can be pulled all the way up to the top of a tree in the xylem vessel during transpiration Water has a low viscosity which means that it flows freely because water molecules can easily slide over each other. The structure of water comprises of an oxygen atom bonded to two hydrogen atoms via the sharing of electrons making it covalent. The shape of the molecule is bent and forms an angle of 104.5 degrees, The hydrogen has a slightly positive charge while the oxygen has a slightly negative charge due to the uneven sharing of electrons as oxygen is more electronegative than hydrogen. Pond Skater vs Northern Pike Water is a very important environment for both pond skaters and northern pikes. The pond skaters can stand on water due to the surface tension created by the cohesion between water molecules. Pond Skaters also use water to hunt for food on the surface of the water. The water provides an environment for the pond skater to stay and a source of good. ‘For the pike, water acts as a solvent for providing dissolved oxygen for respiration and survival. Water’s high specific heat capacity, also helps to keep the water temperature stable for survival The high density provides buoyancy for the pike. Ice is less dense than water. This causes ice to float on water which provides insulation for the pike under water Water molecules are made up of one oxygen atom and two hydrogen atoms bonded covalently with the hydrogens separated by a bond angle of 104.5 degrees. The shape of water is bent. The molecule has no overall charge but because Oxygen is more electronegative than H, it pulls the electrons towards itself, giving itself a partial negative charge and the hydrogens partial positive charge, making the water molecules polar. It is capable of forming hydrogen bonds. Hydrogen bonding forms in liquid water as the hydrogen atoms of one water molecule are attracted towards the oxygen atom of a neighboring water molecule Cohesion and surface tensionHydrogen bonds between oh hold molecules together at the water’s surface, creating the surface tension (The property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of its molecules)- Hydrogen bonds are quite weak. High cohesion supports the columns of water in xylems, which facilitates transport. Good solvent for polar substances- Polar molecules are attracted to weak charges on the water molecules. This keeps the individual ion separated. Role- Transport of many It is the solvent for the electrolytes and nutrients needed by the cells, and also the solvent to carry waste material away from the cells. Water has a high specific heat capacity- 4200J of energy will raise 1kg of a substance by 1-degree Celsius and break the bonds between the water molecules. This increases the temperature. Water has a high specific heat capacity. This means that it requires a lot of energy just to raise the temperature of water. These organisms do not undergo a rapid change in temperature when their external environment’s temperature rapidly increased. This also limits temperature fluctuation in oceans. Ice is less dense than water- When water freezes, molecules slow down and move apart. This leads to the water become less dense. This enables ice to float on water and hence, insulates the organisms below the surface of the water High latent heat of vaporization- Large thermal energy is required to change the state of water from a liquid to a gas. This allows for watery environments to maintain stable conditions. Sweating has a cooling effect! The water absorbs heat energy and evaporates, leaving the skin cooler. Water is reactive- Water participates in many chemical reactions in metabolism. Water is required to break certain chemical bonds Biological Molecules1. Many biological molecules are macromolecules – Macromolecules are made of polymers Polymers are large molecules made of many smaller repeating subunits Monomers are joined by specific bonds Bonds between monomers are formed by condensation reactions- A condensation reaction is a reaction where the water molecule is removed during bond formation Bonds between monomers are broken by hydrolysis- Addition of water to break bonds CarbohydratesGeneral form Cx(H2O)y There are three types of carbohydrates- Monosaccharides, disaccharides and polysaccharides MONOSACCHARIDES Monosaccharides are simple sugars (CH2O)n (n is 1-7) Monosaccharides are energy sources and are used as a building block of carbohydrate polymers All monosaccharides taste sweet Glucose, Fructose and Galactose Note (pentose-5 carbons and hexose 6 carbons) Examples of monosaccharides- Ribose - a basic building block of RNA (ribonucleic acid), while deoxyribose is a basic building block of DNA (deoxyribonucleic acid)….. Glucose Two isomers of glucose are alpha glucose and beta glucose Alpha glucose- The hydroxyl group of carbon 1 is on the opposite side of the ring from carbon 6 (CH2OH) group while in beta glucose, the hydroxyl group of carbon 1 is found on the same side of the ring as carbon 6 (CH2OH) group Properties of monosaccharides- Soluble in water due to the presence of hydroxyl group that are able to form hydrogen bonds with water. Also considered to be polar molecules and sweet DISACCHARIDES Formed from the joining together of two monosaccharides These monosaccharides combine in a condensation reaction Bond between two monosaccharides is known as a glycosidic bond or glycosidic linkage This bond is usually formed between carbon 1 and number 4 of the bonding monosaccharides 1-4 glycosidic link Disaccharides are polar due to the presence of hydroxyl groups Maltose is formed from the joining together of two alpha glucose molecules Sucrose-One alpha glucose and one beta fructose combine to form sucrose The bond occurs between carbon 1 of glucose and carbon 2 of fructose This is known as an alpha 1 - beta 2 glycosidic bond. Importance of sucrose-Can be used for respiration in humans. Sucrase breaks down sucrose into fructose and glucose. Fructose is rearranged to form glucose Sucrose is also transported in the phloem of plants. This is very useful in transporting the products of photosynthesis and sucrose is unreactive, and will not be used on the way to its destination Testing for reducing sugars All mono and di are reducing sugars Benedict’s is used to test for reducing sugar. Benedict’s solution contains copper ii sulphate which is dark blue. When it reacts with a reducing sugar, Cu2+ is reduced to Cu1+. The Cu1+ then forms copper 1 oxide which is a brick red insoluble solid Sucrose is a non-reducing sugar TO test for non-reducing sugars, hydrochloric acid is added to the sample and heated. The acid breaks the glycosidic link, splitting it into its respective monomers. Add an alkali to neutralize the acid. Run the benedict’s test again Polysaccharides- The monomers in many types of polysaccharides are linked by 1-4 glycosidic link and 1-6 glycosidic link 1-4 are present in straight chains of linked monosaccharides 1,6-glycosidic bonds are present at the branches of monosaccharide chain Polysaccharides are non-polar due to the hydroxyl groups responsible for generating polarity, being used up in glycosidic bond STARCH-Used for energy storage in plants The monomer of starch is alpha glucose Two components- amylose and amylopectin Amylose is made of millions of alpha glucose molecules joined by !-1,4 glycosidic bonds. Due to the glycosidic bonds, the amylose chain coils which gives it a more compact shape Amylopectin Made up of millions of alpha glucose molecules joined by alpha 1-4 glycosidic bonds in a straight chain and alpha 1-6 glycosidic bonds which creates branches Amylopectin is less compact than amylose due to branching Starch as a storage molecule Starch is insoluble and inactive - this makes it a useful energy store as it does not influence the water potential Branched and coiled- which makes it dense and compact and hence it takes up less space Polymer of alpha glucose- can be obtained for respiration readily Too large to cross plasma membrane Testing for starch- Iodine reagent is formed by adding iodine to potassium iodide to form triiodide When the orange brown iodine reagent is added to a starch solution: Iodine fit into the amylose coil, resulting in a blue-black colour Glycogen- Glycogen is a polysaccharide used for energy storage in animals Monomer- Alpha Glucose Alpha glucose is joined by alpha -1,4 glycosidic linkages. Many alpha glucose molecules are also joined by alpha 1-6 glycosidic linkages, to form branches Differences between glycogen and starch Glycogen has more alpha1-,6 linkages and is therefore more branched and less dense than Starch- More compact Glycogen is also more soluble than starch. Glycogen hydrolysis occurs rapidly to release energy quickly. This is because animals require more energy than plants, and energy needs to be readily available Glycogen is stored in muscles and liver CELLULOSE- Used for structural support. Cell walls of plants- Made up of beta glucose lined by eta 1-4 glycosidic links Due to rotation and hydrogen bonding, cellulose does not coil Hydrogen bonds add strength Hydrogen bonds form between rotated residues on neighbouring chains