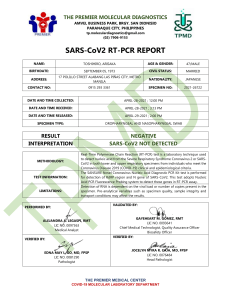
SPECIMEN Patient No: Collected Date: 09/18/2022 Final Report: 09/19/2022 Sample ID number Received Date: 09/192022 PHYSICIAN PATIENT Facility: College Park Medical Center NAME: AKINKOYE, AYODEJI DOB: 10/09/1994 GENDER: MALE CLIA Number:21D2136938 Primary Care: Med Ped Healthcare Urgent Care: Express Care Attending: Dr Jamal Fadul Address: 4701 Melbourne Place, College Park, Maryland 20740 Tel: (301)345-4400 DIAGNOSIS Nasopharynx - Swab: Positive for SARS-CoV-2 (COVID-19), See Comment VIRAL STRAIN METHOD RESULT SARS-CoV-2 CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel Detected The CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel is a real-time RT-PCR test intended for the qualitative detection of nucleic acid from the 2019-nCoV in respiratory specimens (such as nasopharyngeal or oropharyngeal swabs) collected from individuals who meet 2019-nCoV clinical and/or epidemiological criteria (e.g., clinical signs and symptoms associated with 2019-nCoV infection, contact with a probable or confirmed 2019-nCoV case, history of travel to geographic locations where 2019-nCoV cases were detected, or other epidemiologic links for which 2019-nCoV testing may be indicated as part of a public health investigation). Testing in the United States is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. § 263a, to perform high complexity tests. The CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time PCR Diagnostic Panel is only for use under the FDA’s Emergency Use Authorization (EUA). Positive results are indicative of active infection with 2019-nCoV but do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease. Laboratories within the United States and its territories are required to report all positive results to the appropriate public health authorities. Negative results do not preclude 2019-nCoV infection and should not be used as the sole basis for treatment or other patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information. Negative results do not preclude 2019-nCoV infection and should not be used as the sole basis for treatment or other patient management decisions. Optimum specimen types and timing for peak viral levels during infections caused by 2019-nCoV have not been determined. Collection of multiple specimens (types and time points) from the same patient may be necessary to detect the virus. Possible causes of indeterminate results include, but are not limited to: - suboptimal sample collection, improper specimen handling or shipping, low quantity of viral target in the clinical specimen approaching limit of Detection (LOD) of the assay, the presence of RT-PCR inhibitors in the sample. If the virus mutates in the rRT-PCR target region, 2019-nCoV may not be detected or may be detected less predictably. For additional information, please refer to the CDC Fact Sheet for Patients located online at: https://www.cdc.gov/coronavirus/2019-ncov/downloads/Factsheet-for-Patients-2019-nCoV.pdf College Park Medical Center Tel: (301)345-4400 4701 Melbourne Place, College Park, Maryland 20740 Authorized by Douglas Toal, Ph.D. (ABMM), Jamal Fadul, MD, Oussama Mhamdi, Ph.D