Polymer Properties Exercise: UV Spectroscopy & Beer-Lambert Law
advertisement
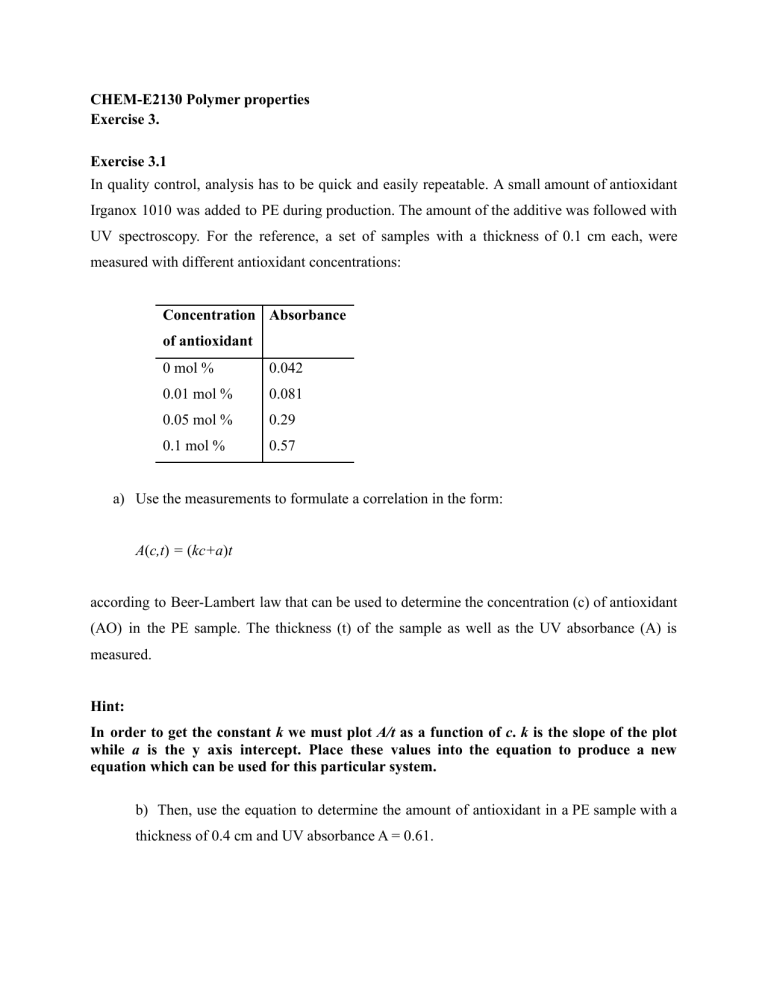
CHEM-E2130 Polymer properties Exercise 3. Exercise 3.1 In quality control, analysis has to be quick and easily repeatable. A small amount of antioxidant Irganox 1010 was added to PE during production. The amount of the additive was followed with UV spectroscopy. For the reference, a set of samples with a thickness of 0.1 cm each, were measured with different antioxidant concentrations: Concentration Absorbance of antioxidant 0 mol % 0.042 0.01 mol % 0.081 0.05 mol % 0.29 0.1 mol % 0.57 a) Use the measurements to formulate a correlation in the form: A(c,t) = (kc+a)t according to Beer-Lambert law that can be used to determine the concentration (c) of antioxidant (AO) in the PE sample. The thickness (t) of the sample as well as the UV absorbance (A) is measured. Hint: In order to get the constant k we must plot A/t as a function of c. k is the slope of the plot while a is the y axis intercept. Place these values into the equation to produce a new equation which can be used for this particular system. b) Then, use the equation to determine the amount of antioxidant in a PE sample with a thickness of 0.4 cm and UV absorbance A = 0.61. Beer Lambert equation:




