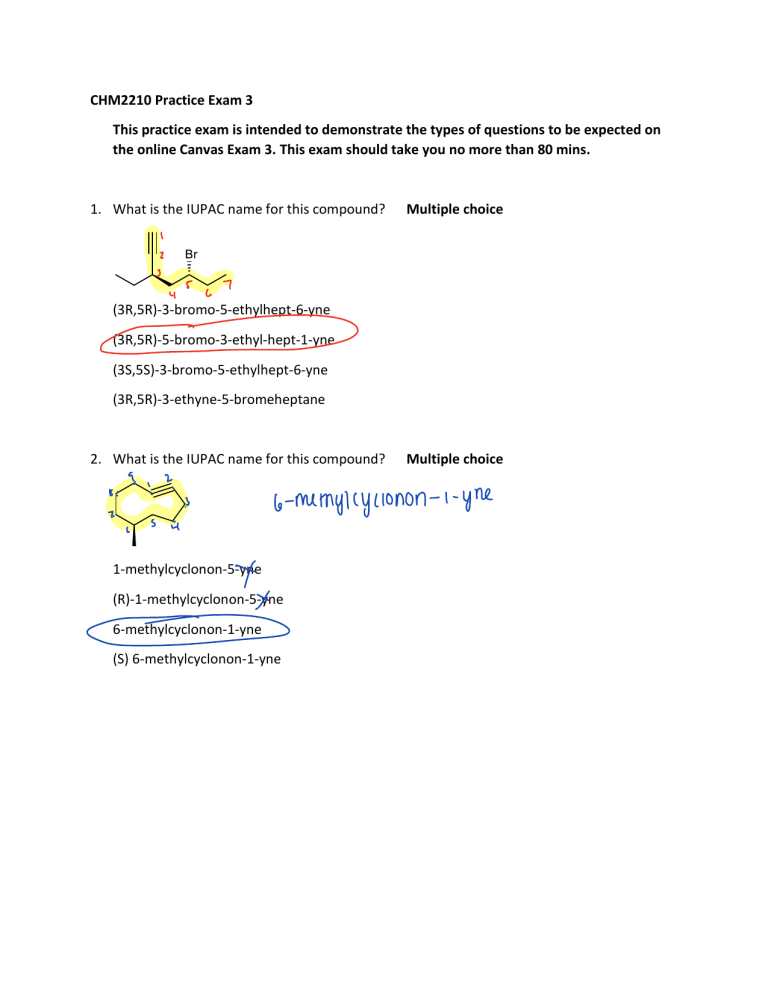
CHM2210 Practice Exam 3 This practice exam is intended to demonstrate the types of questions to be expected on the online Canvas Exam 3. This exam should take you no more than 80 mins. 1. What is the IUPAC name for this compound? Multiple choice 5 a 7 y (3R,5R)-3-bromo-5-ethylhept-6-yne (3R,5R)-5-bromo-3-ethyl-hept-1-yne (3S,5S)-3-bromo-5-ethylhept-6-yne (3R,5R)-3-ethyne-5-bromeheptane 2. What is the IUPAC name for this compound? ii Multiple choice 6 memylcyclonon 1-methylcyclonon-5-yne x (R)-1-methylcyclonon-5-yne 6-methylcyclonon-1-yne (S) 6-methylcyclonon-1-yne i ym 3. Select the line-angle formula that represents 2,5,5-trimethylhept-1-en-3-yne. 0 I 4. Select the structure that represents (R)-4-ethylcyclooct-1-yne. a 5. Rank the following radicals in terms of their stability (least stable to most stable). Fill in multiple blanks least stable B __ < __ C A < __ most stable 6. Rank the C–H bonds indicated below in terms of their bond dissociation enthalpy (BDE). Fill in multiple blanks. CI CI Lowest C __ < __ < B A __ Highest 7. Choose the expected product of the reaction sequence below T HN s ii fit Ii 8. How many equivalents of NaNH2 are required for the first step of this reaction? Numerical Answer: J f a'timpani HD H 9 3 O 9. Which C–H bond below is weaker (in terms of bond strength), A or B? Pull down menu selection: A or B 10. Consider the reaction shown below. Complete the two propagation steps by predicting the structures of A–C. Pull down menu selections: Select compound A from List 1. (Answers: 1a, 1b, 1c, 1d) Select compound B from List 2. (Answers: 2a, 2b, 2c, 2d) Select compound C from List 2. (Answers: 2a, 2b, 2c, 2d) 11. Select a reasonable reaction intermediate to the following reaction 12. Select the expected products for each of the following reactions. 12a.) 12b) 12c) 12d) 13. How many different monochlorination products may be formed in the reaction below? Ignore stereochemistry. Numerical answer: 14. Select the expected products for the following reactions. 15. Propose a synthesis for the target molecules shown below. You must start your synthetic route using the compounds shown; from there, you can use any other materials/reagents you need. Use the pull-down menus to select the reagents/conditions. You must select one option for each of the pull-down menus below. List of Reagents/Conditions (Dropdown menu): Br2, CH2Cl2 O3 Br2, H2O (CH3)2S NaNH2, NH3 HCC- Na+ (sodium acetylide) H2O BH3, THF H2, Lindlar NaOH, H2O2 Na, NH3 Hg(OAc) 2, THF/H2O H2, Pd NaBH4, H2O HgSO4, H2SO4, H2O CH3Br HB(sia)2, THF CH3CH2Br OsO4, H2O2, H2O H2O, H2SO4 (cat.) Synthesis 1 (4 steps): Synthesis 2 (3 steps): CHM2210 Practice Exam 3 This exam should take no more than 80 mins. 1. What is the IUPAC name for this compound? Multiple choice (3R,5R)-3-bromo-5-ethylhept-6-yne (3R,5R)-5-bromo-3-ethyl-hept-1-yne (3S,5S)-3-bromo-5-ethylhept-6-yne (3R,5R)-3-ethyne-5-bromeheptane 2. What is the IUPAC name for this compound? 1-methylcyclonon-5-yne (R)-1-methylcyclonon-5-yne 6-methylcyclonon-1-yne (S) 6-methylcyclonon-1-yne Multiple choice 3. Select the line-angle formula that represents 2,5,5-trimethylhept-1-en-3-yne. Multiple choice, Answer: 2 4. Select the structure that represents (R)-4-ethylcyclooct-1-yne. Multiple choice, Answer: 2 5. Rank the following radicals in terms of their stability (least stable to most stable). Fill in multiple blanks least stable __ < __ < __ most stable least stable B < A < C most stable 6. Rank the C–H bonds indicated below in terms of their bond dissociation enthalpy (BDE). Fill in multiple blanks. Lowest __ < __ < __ Lowest C<A<B highest BDE 7. Choose the expected product of the reaction sequence below Multiple choice, Answer: 4 8. How many equivalents of NaNH2 are required for the first step of this reaction? Numerical answer: 3 9. Which C–H bond below is weaker (in terms of bond strength), A or B? Pull down menu selection: A or B 10. Consider the reaction shown below. Complete the two propagation steps by predicting the structures of A–C. Pull down menu selections: Select compound A from List 1. (Answers: 1a, 1b, 1c, 1d) Select compound B from List 2. (Answers: 2a, 2b, 2c, 2d) Select compound C from List 2. (Answers: 2a, 2b, 2c, 2d) 11. Select a reasonable reaction intermediate to the following reaction Multiple choice Answer: 1 12. Select the expected products for each of the following reactions. Pull down menu Select A from the compounds shown above. Answer: 3 Select B from the compounds shown above. Answer: 2 Pull down menu Select A from the compounds shown above. Answer: 1 Select B from the compounds shown above. Answer: 4 Pull down menu Select A from the compounds shown above. Answer: 3 Select B from the compounds shown above. Answer: 1 Pull down menu Select A from the compounds shown above. Answer: 2 Select B from the compounds shown above. Answer: 4 13. How many different monochlorination products may be formed in the reaction below? Ignore stereochemistry. Numerical answer: 3. 14. Select the expected products for the following reactions. Pull down menu Select A from the compounds shown above. Answer: 1 Select B from the compounds shown above. Answer: 2 15. Propose a synthesis for the of the target molecules shown below. You must start your synthetic route using the compounds shown; from there, you can use any other materials/reagents you need. Use the pull-down menus to select the reagents/conditions. You must select one option for each of the pull-down menus below. TWO SEPARATE SYNTHESIS PROBLEMS. List of Options (Dropdown menu): Br2, CH2Cl2 O3 Br2, H2O (CH3)2S NaNH2, NH3 HCC- Na+ (sodium acetylide) H2O BH3, THF H2, Lindlar NaOH, H2O2 Na, NH3 Hg(OAc) 2, THF/H2O H2, Pd NaBH4, H2O HgSO4, H2SO4, H2O CH3Br HB(sia)2, THF CH3CH2Br OsO4, H2O2, H2O H2O, H2SO4 (cat.) Synthesis 1: 4 Pull-downs: Step a: Br2, CH2Cl2 Step b: NaNH2, NH3 Step c: CH3Br Step d: H2, Pd Synthesis 2: 3 Pull-downs: Step a: Br2, CH2Cl2 Step b: NaNH2, NH3 Step c: CH3CH2Br


