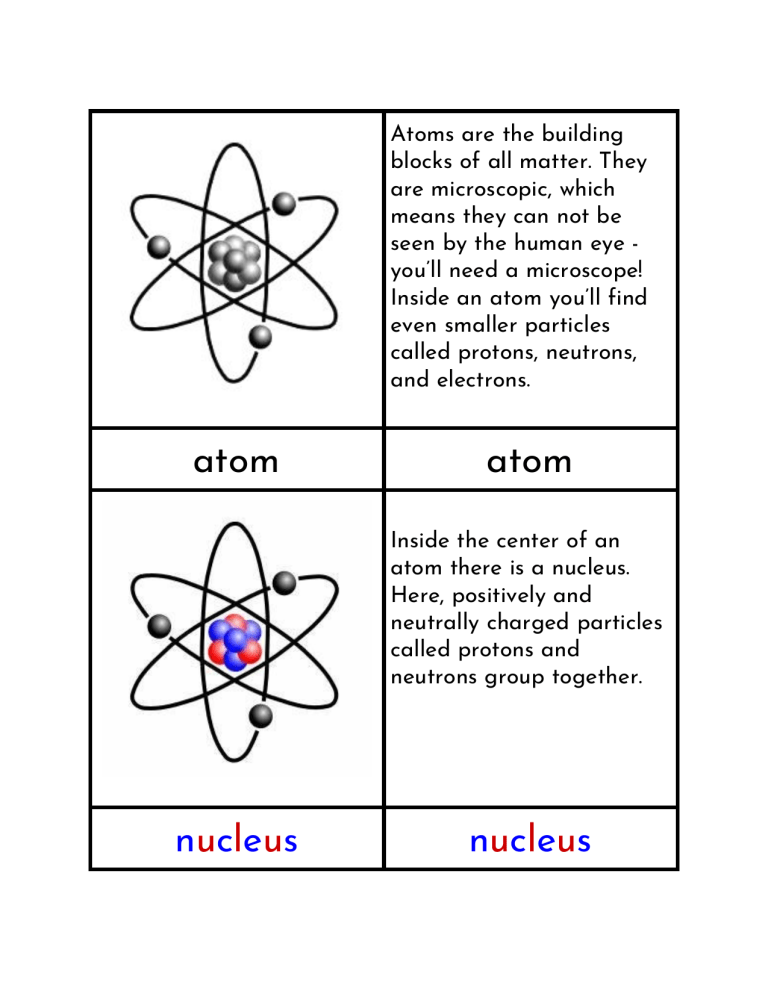
Atoms are the building blocks of all matter. They are microscopic, which means they can not be seen by the human eye you’ll need a microscope! Inside an atom you’ll find even smaller particles called protons, neutrons, and electrons. atom atom Inside the center of an atom there is a nucleus. Here, positively and neutrally charged particles called protons and neutrons group together. nucleus nucleus A proton is a positively charged particle found in the nucleus of an atom. When there is an equal number of protons and electrons, an atom will have no charge. proton proton A neutron is a neutrally charged particle found in the nucleus of an atom. Unlike protons and electrons, it has no charge. Neutrons can be found in the atoms of all elements except Hydrogen. neutron neutron An electron is a negatively charged particle found orbiting outside the atom’s nucleus. When there is an equal number of protons and electrons, an atom will have no charge. electron electron atom nucleus proton neutron MindfulTeaching Parts of an Atom Three/Four Part Cards electron #bemindful





