VSEPR Theory Practice Worksheet
advertisement

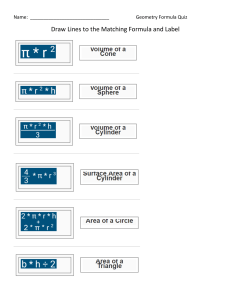
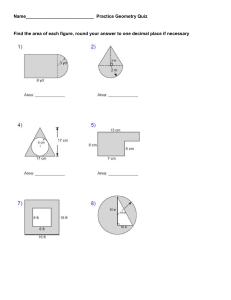
Name: _____________________________________________________ Date: ____________________ PRACTICE VSEPR Theory _________________________________________________________________________________________________________________________________ Directions: Reference the worksheet entitled PRACTICE: Lewis Structures. Assign the electron pair geometry, molecular geometry, hybridization, and bond angle with respect to the central atom(s). Also, determine if the molecule is polar or nonpolar, and indicate the predominant type of intermolecular force exhibited by the molecule. A. Standard/Deficient Octets Problem Number Chemical Formula 1 CH4 2 BBr3 3 PCl3 6 CO2 10 HCN AP Chemistry Electron Pair Geometry Molecular Geometry Hybridization Bond Angle Polarity IMF Unit 5: Bonding, States of Matter, and Solutions B. Multiple Central Atoms Problem Number 2 3 Chemical Formula Electron Pair Geometry Molecular Geometry Hybridization Bond Angle Polarity IMF Bond Angle(s) Polarity IMF C-1: C-1: C-1: C-1: C-2: C-2: C-2: C-2: C-1: C-1: C-1: C-1: C-2: C-2: C-2: C-2: C2H4 C2H2 C. Expanded Octets Problem Number Chemical Formula 1 PCl5 N/A 2 ICl3 N/A 3 SF6 N/A 4 XeF4 N/A AP Chemistry Electron Pair Geometry Molecular Geometry Hybridization Unit 5: Bonding, States of Matter, and Solutions D. Ions Problem Number Chemical Formula 1 NH4+ Electron Pair Geometry Molecular Geometry Hybridization Bond Angle Polarity N/A E. Resonance Problem Number Chemical Formula 1 NO2- Electron Pair Geometry Molecular Geometry Hybridization Bond Angle Polarity N/A F. Oxyacids Problem Number Chemical Formula Electron Pair Geometry 1 Molecular Geometry Hybridization Bond Angle N: N: N: N: O*: O*: O*: O*: Polarity * HNO2 AP Chemistry Unit 5: Bonding, States of Matter, and Solutions Problem Number Chemical Formula * 2 Electron Pair Geometry Molecular Geometry Hybridization Bond Angle C: C: C: C: O*: O*: O*: O*: Polarity * H2CO3 AP Chemistry Unit 5: Bonding, States of Matter, and Solutions