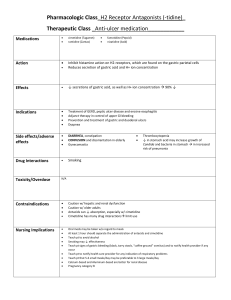
Lilley: Pharmacology and the Nursing Process, 9th Edition Chapter 50: Acid-Controlling Drugs Key Points One stomach condition requiring drug therapy is hyperacidity, or excessive acid production. Left untreated, hyperacidity can lead to serious conditions such as acid reflux, ulcer disease, esophageal damage, and even esophageal cancer. Anatomy, Physiology, and Disease Overview The stomach secretes many substances (hydrochloric acid, pepsinogen, mucus, bicarbonate, intrinsic factor, and prostaglandins). The gastric glands are highly specialized secretory glands composed of several different types of cells: parietal, chief, mucous, endocrine, and enterochromaffin. Parietal cells produce and secrete hydrochloric acid (HCl). They are the primary site of action for many of the drugs used to treat acid-related disorders. Chief cells secrete pepsinogen. Pepsinogen is a proenzyme (enzyme precursor) that becomes pepsin when activated by exposure to acid. Pepsin breaks down proteins and is therefore referred to as a proteolytic enzyme. Mucous cells are mucus-secreting cells that are also called surface epithelial cells. The secreted mucus serves as a protective coating against the digestive action of HCl and digestive enzymes. In acid-related disorders, there is an impairment of the balance among the substances secreted by the stomach. The most harmful of the acid-related diseases involve hypersecretion of acid and include peptic ulcer disease and esophageal cancer. The most common condition is mild to moderate hyperacidity. Hyperacidity is often associated with gastroesophageal reflux disease (GERD). This is the tendency of excessive and acidic stomach contents to back up, or reflux, into the lower (and even upper) esophagus. Over time, this condition can lead to more serious disorders such as erosive esophagitis and Barrett esophagus, a precancerous condition. HCl maintains the environment of the stomach at a pH of 1 to 4. This acidity aids in digestion and also serves as one of the body’s defenses against microbial infection via the gastrointestinal (GI) tract. Several substances stimulate HCl secretion by the parietal cells, such as food, caffeine, chocolate, and alcohol. In moderation, any of these is usually not problematic. Excessive consumption of large, fatty meals or alcohol, as well as emotional stress, may result in hyperproduction of HCl and lead to hypersecretory disorders such as peptic ulcer disease. Peptic ulcer disease is a general term for gastric or duodenal ulcers that involve digestion of the GI mucosa by the enzyme pepsin. Copyright © 2020 Elsevier Inc. All Rights Reserved. Key Points 50-2 Because the process of ulceration is driven by the proteolytic (protein breakdown) actions of pepsin together with the caustic effects of HCl, peptic ulcer disease and related problems are also referred to by the more general term acid-peptic disorders. Helicobacter pylori (H. pylori) is implicated in the pathophysiology of peptic ulcer disease. The prevalence of H. pylori as measured by serum antibody tests is approximately 40% to 60% for patients older than 60 but only 10% for those younger than 30 years of age. H. pylori is found in the GI tracts of roughly 90% of patients with duodenal ulcers and 70% of those with gastric ulcers. First-line therapy includes a 10- to 14-day course of a proton pump inhibitor and the antibiotics clarithromycin and either amoxicillin or metronidazole. Stress-related mucosal damage is an important issue for critically ill patients. Stress ulcer prophylaxis is used in almost all patients in intensive care units (ICUs) and some on general medical surgical units. GI lesions are common in ICU patients, especially in the 24 hours after admission. Factors include decreased blood flow, mucosal ischemia, hypoperfusion, and reperfusion injury. Guidelines suggest that all such patients receive either a histamine receptor–blocking drug or a proton pump inhibitor. Pharmacology Overview Antacids Antacids are basic compounds used to neutralize stomach acid. Most commonly they are nonprescription salts of aluminum, magnesium, calcium, and/or sodium. Many antacid preparations contain the antiflatulent drug simethicone, which reduces gas and bloating. Many aluminum- and calcium-based formulations also include magnesium, which not only contributes to the acid-neutralizing capacity but also counteracts the constipating effects of aluminum and calcium. Antacids work primarily by neutralizing gastric acidity. They do not prevent the overproduction of acid but instead help to neutralize acid secretions. It is also believed that antacids promote gastric mucosal defensive mechanisms, especially at lower dosages. The primary drug effect of antacids is the reduction of the symptoms associated with various acid-related disorders, such as pain and reflux. Antacids are indicated for the acute relief of symptoms associated with peptic ulcer, gastritis, gastric hyperacidity, and heartburn. Adverse effects of antacids are limited. Magnesium preparations, such as milk of magnesia, can cause diarrhea. Aluminum- and calcium-containing formulations can cause constipation. Magnesium-aluminum combination antacids are used to prevent the adverse effects of constipation and diarrhea. Some of the more serious concerns with antacids include acid rebound, hypercalcemia, milk-alkali syndrome, and metabolic alkalosis. Combination products containing both magnesium and aluminum may have fewer adverse effects than either type of antacid by itself. The net effect is a balancing of both adverse effects and fewer problems with altered bowel patterns. Copyright © 2020 Elsevier Inc. All Rights Reserved. Key Points 50-3 An adverse effect more common with the calcium-containing products is rebound hyperacidity, in which the patient has hyperacidity when antacid use is discontinued. Cautious use of antacids high in sodium is recommended in patients who have heart failure, hypertension, or other cardiac diseases or who require sodium restriction. Many drug interactions occur with the acid-controlling drugs due to alteration of oral dosage forms, and so other medications are to be avoided within 1 to 2 hours of taking an antacid. Antacids are sometimes to be avoided when other acid-controlling drugs are taken. Four basic mechanisms by which antacids cause interactions include adsorption of other drugs to antacids, which reduces the ability of the other drug to be absorbed into the body; chelation, which is the chemical inactivation of other drugs that produces insoluble complexes; increased stomach pH, which increases the absorption of basic drugs and decreases the absorption of acidic drugs; and increased urinary pH, which increases the excretion of acidic drugs and decreases the excretion of basic drugs. Significant patient harm may ensue when the quinolone antibiotics (ciprofloxacin, levofloxacin, moxifloxacin) are given with antacids. Some of the more serious concerns with antacids include acid rebound, hypercalcemia, milk-alkali syndrome, and metabolic alkalosis. H2 Receptor Antagonists H2 receptor antagonists, also known as H2RAs and H2 receptor blockers, are the prototypical acid-secretion antagonists. They are H2 blockers that bind to and block histamine receptors located on parietal cells. This blockade renders these cells less responsive to stimuli and thus decreases their acid secretion. Up to 90% inhibition of acid secretion can be achieved. The effect of reduced hydrogen ion secretion from the parietal cells results in an increase in the stomach pH and relief of symptoms associated with hyperacidity-related conditions. H2 receptor antagonists are used for GERD, peptic ulcer disease, and erosive esophagitis; adjunct therapy in the control of upper GI tract bleeding; and treatment of pathologic gastric hypersecretory conditions such as Zollinger-Ellison syndrome. The H2 receptor antagonists include the drugs cimetidine, ranitidine, famotidine, and nizatidine. Ranitidine (Zantac) has many fewer drug interactions compared to cimetidine and has become the most widely used H2 receptor antagonist. Cimetidine carries a higher risk of drug interactions than ranitidine, famotidine, and nizatidine, especially in elderly. Cimetidine binds enzymes of the hepatic cytochrome P450 microsomal oxidase system, liver enzymes that metabolize many different drugs. By inhibiting the metabolism of drugs metabolized via this pathway, it may raise the blood concentrations of certain drugs. All drugs in this class are available over the counter. Proton Pump Inhibitors Drugs used for the treatment of acid-related disorders are the proton pump inhibitors (PPIs). These include lansoprazole (Prevacid), omeprazole (Prilosec), rabeprazole Copyright © 2020 Elsevier Inc. All Rights Reserved. Key Points 50-4 (AcipHex), pantoprazole (Protonix), esomeprazole (Nexium), and dexlansoprazole (Dexilant). Zegerid is a combination of omeprazole and sodium bicarbonate. The PPIs bind directly to the hydrogen-potassium-ATPase pump mechanism and irreversibly inhibit the action of this enzyme, resulting in total blockage of hydrogen ion secretion. PPIs block the final step in the acid production pathway, the hydrogenpotassium-ATPase pump, and they block all acid secretion. PPIs are currently indicated as first-line therapy for erosive esophagitis, symptomatic GERD that is poorly responsive to other medical treatment such as therapy with H2 receptor antagonists, short-term treatment of active duodenal ulcers and active benign gastric ulcers, gastric hypersecretory conditions (e.g., Zollinger-Ellison syndrome), nonsteroidal antiinflammatory drug (NSAID)–induced ulcers, and for stress ulcer prophylaxis. All PPIs can be used in combination with antibiotics to treat H. pylori infections. When omeprazole is given with clopidogrel, there is some concern of a decrease in clopidogrel’s effectiveness due to the fact that clopidogrel is dependent on its conversion to an active metabolite by the CYP-450 enzyme system, specifically CYP2C19. PPIs are generally well tolerated. The U.S. Food and Drug Administration issued a warning in 2010 regarding long-term use of high-dose PPIs, which has been associated with Clostridium difficile infections; risk of wrist, hip, and spine fractures; and pneumonia. In 2011, depletion of magnesium was added to the warning. PPIs may increase serum levels of diazepam and phenytoin and there may be an increased chance of bleeding in patients who are taking both a PPI and warfarin. Miscellaneous Acid-Controlling Drugs There are a few other acid-controlling drugs that are unique in terms of their mechanisms and other features, including sucralfate, misoprostol, and simethicone. Sucralfate is used for the treatment of peptic ulcer disease and stress-related ulcers. It binds to tissue proteins in the eroded area and prevents exposure of the ulcerated area to stomach acid. Misoprostol (Cytotec) is a synthetic prostaglandin analog that inhibits gastric acid secretion and is used to prevent NSAID-related ulcers. Simethicone (Mylicon) is used to reduce the discomforts of gastric or intestinal gas (flatulence) and aid in its release via the mouth or rectum. Nursing Process Before an acid-controlling drug is given, perform a thorough patient assessment with attention to past and present medical history and with special focus on GI tract–related disorders and signs and symptoms of ulcer disease and GERD. Since acid-controlling drugs have many interactions, underscore the importance of obtaining a medication history about prescription drugs, over-the-counter drugs, and herbals. The high sodium content of various antacids may lead to exacerbation of cardiac problems, renal dysfunction, and fluid-electrolyte problems. Copyright © 2020 Elsevier Inc. All Rights Reserved. Key Points 50-5 With quinolone antibiotics, there may be serious harm if given with antacids because of a 50% reduction in antibiotic absorption. For patients using H2 receptor antagonist drugs, assess renal and liver function and level of consciousness. Elderly patients react to these drugs with more disorientation and confusion. Do not administer drugs such as cimetidine and famotidine simultaneously with antacids. Since there are documented concerns about the use of PPIs and development of osteoporosis, thoroughly assess patients for any history of this disorder. The use of simethicone and sucralfate requires assessment of the patient’s bowel patterns and bowel sounds and evaluation for abdominal distention and rigidity. When giving acid-controlling drugs, instruct the patient to thoroughly chew the chewable tablets and to thoroughly shake liquid forms. Antacids need to be given with at least 8 oz of water to enhance absorption, except for newer forms that are rapidly dissolving drugs. For all of these H2 receptor antagonists, monitor blood pressure readings as needed during intravenous infusion because of the risk of hypotension. Continue to monitor the patient for GI tract bleeding with the diagnosis of ulcers or GI irritation. Report any blood in the stools or the occurrence of black, tarry stools or hematemesis. With PPIs, give lansoprazole oral dosage forms as ordered and with fluids. Monitor for abdominal pain, distention, and abnormal bowel sounds. Always double-check the names and dosages of these drugs to ensure that they are not confused with similarly named drugs. Therapeutic response to the administration of antacids, H2 receptor antagonists, PPIs, and other related drugs includes the relief of symptoms associated with peptic ulcer, gastritis, esophagitis, gastric hyperacidity, or hiatal hernia (i.e., decrease in epigastric pain, fullness, and abdominal swelling). Adverse effects range from constipation or diarrhea to nausea, vomiting, abdominal pain, and hypotension. Milk-alkali syndrome, acid rebound, hypercalcemia, and metabolic alkalosis are known complications associated with the various antacids. Copyright © 2020 Elsevier Inc. All Rights Reserved.