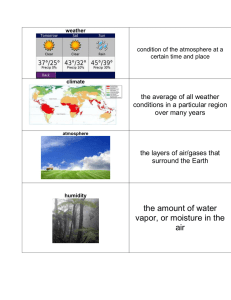
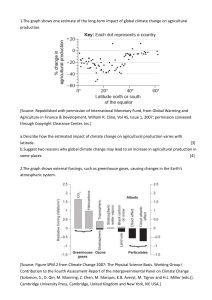
1 CHAPTER#1 AIR QUALITY: DEFINITIONS CHARACTERISTICS& PERSPECTIVES 2 What is Atmosphere? An atmosphere is a layer of gas or layers of gases that envelope a planet, and is held in place by the gravity of the planetary body. The atmosphere of Earth is composed of nitrogen (78%), oxygen (21%), argon (0.9%), carbon dioxide (0.04%) and trace gases. 3 Biosphere is the zone of life on Earth 4 CLASSIFICATION OF ATMOSPHERE IN LAYERS 5 PRESSURE VARIANCE 6 TEMPERATURE VARIATION • WHEN THE ALTITUDE INCREASES, THE TEMPERATURE DECREASES • JABAL AKTAR IS COOLER THAN BIRKAT ALMOUZ • HIMALAYAS [8824 M ] IS MUCH COOLER THAN ANY OTHER PLACE IN THE WORLD [ TEMPERATURE IS -60 DEGREE CELSIUS] 7 COMPOSITION OF CLEAN AIR 8 DEFINITION OF AIR POLLUTION 9 REASONS FOR AIR POLLUTION 10 11 IDENTIFYING AIR POLLUTION 12 13 14 15 16 17 Natural Fog 18 19 Pollen Grains 20 Examples 21 22 23 24 An Illinois coal is burned at a rate of 1.00 kg per second. If the analysis of the coal reveals a sulfur content of 3.00 %, what is the annual rate of emission of SO2? Sulfur dioxide (SO2) Sulfur in Sulfur dioxide (ash) MASS BALANCE: Sin = Sash + SSO2 Sin = 1 kg/s x 0.030 = 0.030 kg/s Sin = 9.46 x 105 kg/yr Sash = (0.05)(9.46 x 105 kg/yr) = 4.73 x 104 kg/yr SSO2 = Sin – Sash = 9.46 x 105 – 4.73 x 104 SSO2 = 8.99 x 105 kg/yr SSO2 = 8.99 x 105 kg/yr (64 SO2/32 S) SSO2 = 1.80 x 106 kg/yr Determine whether or not a pulverized coal, dry bottom, wall-fired boiler using bituminous coal at power plant rate at 61 MW meets the NSPS for SO2.The power plant burns bituminous coal with a sulfur content of 1.8% and ash content of 6.2 %. The coal has a heating value of 14,000 Btu/lb. the boiler efficiency is 35%. Use the emission factors to estimate the emissions. Assume the efficiency of SO2 control is 85% . Coal firing rate = 61 MW / 0.35 = 174.3 x 106 W Mass of coal burned = 174.3 x 106 J/s (3600 s) (1 Btu/1054.4 J) = 5.95 x 108 Btu/hr Using the EPA emission factor of 38S for bituminous coal: Uncontrolled SO2 emission rate = 38 (1.8) = 68.4 lbm ton coal Check emission rate: Estimated SO2 emission rate = 68.4 lb/ton coal(5.95 x 108 Btu)(1 ton/2000lb)(0.15) 14,000 Btu/lb = 218.05 lbm SO2 emission rate (per million Btu) = 218.05 lb/(5.95 x 108 Btu)(106) = 0.37 lb/million Btu Comment: meets the standard 1.2 lb/million Btu but not the 90% reduction requirement 32 33 34 35 36 37 38 39 40 41 AIR POLLUTION EPISODES 42 EPISODES • EPISODE – used as a refined form of the word disaster/ incident. • Indeed it was the shock of these disasters that stimulated the first modern legislative action to control of air pollutants. • Careful study of different known episodes reveal that all of the incidents had something in common. AIR POLLUTION EPISODES • CRUCIAL INGREDIENTS FOR AN EPISODE TO HAPPEN: – Large number of population sources – A restricted air volume – Failure of officials to recognize that anything is wrong – The presence of water droplets of the right size Source: Goldsmith 1968 AIR POLLUTION EPISODES Major air pollution episodes(WHO, 1961) Donora, 1948 London, 1952 Population Weather 12,300 8,000,000 Anticyclone Anticyclone inversion and fog inversion and fog Topography Most probable source of pollutants River valley Industry steel and zinc plants River plain Household and coal burning Major air pollution episodes (WHO, 1961) Donora, 1948 Nature of illnesses Chemical irritation of exposed membranous surfaces # of deaths Time of death Suspected cause of irritation 17 London, 1952 Chemical irritation of exposed membranous surfaces 4000 Began after second day of episode Began after second day of episode Sulfur oxides with particulates Sulfur oxides with particulates Location of Bhopal in India BACKGROUND INFORMATION The Bhopal disaster, also referred to as the Bhopal gas tragedy, was a gas leak incident in India, considered to be the world's worst industrial disaster. Monday, December 3rd, 1984. (28 years ago) BACKGROUND INFORMATION • One of Union Carbide’s Pesticide factories was located in Bhopal, India. • Union Carbide of India Limited (UCIL) was a subsidiary of The Union Carbide Corporation (UCC). • The Factory produced carbanate pesticides. One Component was Methyl Isocyanate (MIC). • A rapidly growing community of roughly 900,000 people. The system that failed The Bhopal Disaster! • 40 tons of deadly gases suddenly burst out into the atmosphere. • Workers fled in panic. • People woke up coughing violently and with eyes burning as if chilli powder had been flung into them. • Neighbouring communities fled in panic • The streets were foul with vomit. Those who fell were trampled by the crowd. • The worst affected were the children: unable to walk and breathe, they simply suffocated and died. The Bhopal Disaster! • Local hospitals were soon overwhelmed with the injured • Over 500,000 people were exposed to methyl isocyanate gas and other chemicals. Contributing Factors Factors leading to the magnitude of the gas leak mainly included problems such as: • storing MIC in large tanks and filling beyond recommended levels, poor maintenance after the plant ceased MIC production at the end of 1984, • safety systems being switched off to save money— including the MIC tank refrigeration system which could have mitigated the disaster severity. • shortcomings in health care and socio-economic rehabilitation. Contributing Factors • use of a more dangerous pesticide manufacturing method, • plant location close to a densely populated area, • undersized safety devices, • Plant management deficiencies were also identified – lack of skilled operators, reduction of safety management, insufficient maintenance, and inadequate emergency action plans. AFTERMATH OF IT ALL • The official immediate death toll was 2,259. The government of Madhya Pradesh confirmed a total of 3,787 deaths related to the gas release. Others estimate 8,000 died within two weeks and another 8,000 or more have since died from gas-related diseases. A government affidavit in 2006 stated the leak caused 558,125 injuries including 38,478 temporary partial injuries and approximately 3,900 severely and permanently disabling injuries. Victims Remain Victims • >Resident Leela was one of those caught by Union Carbide’s cloud of poison gas. • >Her family of six survived, but ever since they have suffered from breathlessness and spells of vomiting. One of her sons has gone blind. All six family members suffer from breathlessness and spells of vomiting. • >Burdened by injury they cannot earn well. The family’s joint income is $30 a month. • For the gas victims of Bhopal every day of the past 28 years has been a struggle against breathlessness, nausea, brain damage, cancers, fevers, numbness, panic attacks, menstrual chaos, monstrous births. Economic Effects • • • • • • loss of jobs (650 permanent jobs were lost) loss of earning capacity of victims business disruptions cost of compensation rehabilitation, and legal costs. "Investment hasn't been coming to Bhopal because of the stigma." NOW * Still in a state Recovering * Almost 30 years later, one out of four babies born in Bhopal is born dead. * Countless people suffer from breathing difficulties, cancer, nerve diseases and infertility. * The ground water is still contaminated. NOW Deteriorating portion of the MIC plant, decades after the gas leak. Contributor to ongoing contamination. NOW Bhopal child born with birth defects GLOBAL EFFECTS OF AIR POLLUTION 63 GLOBAL WARMING What is global warming? Since the Industrial Revolution, the global annual temperature has increased in total by a little more than 1 degree Celsius, or about 2 degrees Fahrenheit. Between 1880—the year that accurate recordkeeping began—and 1980, it rose on average by 0.07 degrees Celsius (0.13 degrees Fahrenheit) every 10 years. Since 1981, however, the rate of increase has more than doubled: For the last 40 years, we’ve seen the global annual temperature rise by 0.18 degrees Celsius, or 0.32 degrees Fahrenheit, per decade. 64 What causes global warming? A: Global warming occurs when carbon dioxide (CO2) and other air pollutants collect in the atmosphere and absorb sunlight and solar radiation that have bounced off the earth’s surface. Normally this radiation would escape into space, but these pollutants, which can last for years to centuries in the atmosphere, trap the heat and cause the planet to get hotter. These heat-trapping pollutants—specifically carbon dioxide, methane, nitrous oxide, water vapor, and synthetic fluorinated gases—are known as greenhouse gases, and their impact is called the greenhouse effect. 65 Earth’s Atmospheric Gases Nitrogen (N2) Oxygen (O2) NonGreenhouse Gases 99% Water (H2O) Carbon Dioxide (CO2) Methane (CH4) Greenhouse Gases 1% The image below describes the Greenhouse effect and the role of greenhouse gases Sun Greenhouse Effect The image shows the concentration of CO2 in the atmosphere over a period of time if emissions continue unaltered. CO2 concentration after 50 years of unrestricted fossil fuel burning (600 ppmv) Present CO2 concentration (386 ppmv) 270 240 210 180 Temp. Proxy CO2 (ppmv) 300 800 600 400 200 Thousands of Years Before Present 0 Households are Big Contributors to Climate Change Of all U.S. greenhouse gas emissions come from households: • Vehicles • Home Heating • Electricity So how can each of us slow global warming now? Reduce our consumption of fossil fuels Because greenhouse gas emissions are tied very closely to our energy consumption, using less fossil fuel based energy puts fewer greenhouse gases into the atmosphere. This will help slow global warming. Mountaintop removal for coal mining near Rawl, West Virginia. 50% of electricity in the United States is produced from coal. ( Average Electricity Emission Factors Region/State South Atlantic North Carolina Virginia West Virginia CO2 lb/kWh CO2 tons/MWh CO2 Metric tons/MWh CH4 lbs/MWh NO2 lbs/MWh 1.35 0.674 0.612 0.0127 0.0207 1.24 0.621 0.563 0.0105 0.0203 1.16 0.582 0.528 0.0137 0.0192 1.98 0.998 0.897 0.0137 0.0316 Kitchen Light Fixture Three 60 Watt Bulbs How much energy are those bulbs using? 1 Wattage of the bulbs (Incandescent bulbs) 60 W 2 # of bulbs Watts Used (Wattage x number of bulbs) 4 Hours used per day (60 x 3) 3 bulbs (CFL bulbs) 18 W 3 3 bulbs 180 W 10 hours a day 18 x3 10 hours a day 54 W 5 Watts Used (#3) x Total Hours/day (#4) 6 Watts hours / year 7 Kilowatt hours / year (#5 x 365 days) (1000Wh = 1kWh) (divide #6 by 1000) (180 x 10) (1800 x 365) (640,800/10 00) 1800 Wh/day 640,800 Wh/ year 640.8 kWh per year 54 x 10 540x365 540 Wh/day 197,100 Wh/day 197,100 1000 197.1 kWh/yr 3 BULB REPLACEMENT EMISSION and COST COMPARISON INCANDESCENT vs. COMPACT FLUORESCENT Incandescents Total kWh for 3 bulb (#7 from above) 640.8 kWh Cost (kWh #7 x $.18) $115.34 CO2 produced @ 1.16 lbs/kWh 743.3 Compact Fluorescents (CFLs) 197 kWh lbs CO2 not emitted by switching 3 bulbs $35.46 228.52 lbs 514.8 (C O2 of incandescents - C O2 of CFLs) Money saved in energy (Cost incandescents - cost of CFLs) $79.88 lbs We can make some simple substitutions Replacing just 1 incandescent light bulb with 1 compact florescent bulb saves about 150 pounds of carbon dioxide per year! If every American household replaced just 5 high-use incandescent bulbs with compact florescent lights we'd collectively save more than $8 billion each year in energy costs and we would prevent the greenhouse gases equivalent to the emissions from nearly 10 million cars. Source: http://www.energystar.gov Be Bulb Smart—Use CFLs Incandescent What’s the difference? Compact Fluorescent 500 lbs. of coal •1,430 lbs. CO2 pollution avoided •$30 saved Small changes really add up Replace your old refrigerator with a new Energy Star: Annual savings: $90; 700 pounds CO2 Set your thermostat down a few degrees in the winter Annual savings: $135; 1400 pounds CO2 Drive JUST 10 fewer miles per week Annual savings: $80; 520 pounds CO2 Wash clothes in cold water only Annual savings: $70; 500 pounds CO2 Reduce your garbage by 10% through greater recycling or reduced packaging Annual savings: 1200 pounds CO2 Caulk and weather-strip around doors and windows Annual savings: $80; 650 pounds CO2 * These are mid-range estimates from published sources; your savings may vary. How is global warming linked to extreme weather? A: Scientists agree that the earth’s rising temperatures are fueling longer and hotter heat waves, more frequent droughts, heavier rainfall, and more powerful hurricanes. What are the other effects of global warming? • Melting Of Polar Ice Caps Rise in Sea Levels Throw global ecosystems out of balance Will endanger several species of animals • Other fallouts include Spread of disease Warmer waters and more hurricanes Increased probability and intensity of droughts and heat waves Economic consequences Loss of Biodiversity Destruction of Ecosystems • Mitigation of global warming involves taking actions to reduce greenhouse gas emissions. Energy efficiency and conservation Urban Planning Building Design Use of passive solar building design, low-energy building, or zero-energy building techniques, using renewable heat sources Transport plug-in hybrid electric vehicles A shift from air transport and truck transport to electric rail transport Increased use of biofuels Carbon Capture And Storage (CCS) • Carbon capture and storage (CCS) is a plan to mitigate climate change by capturing carbon dioxide (CO2) from large point sources such as power plants and subsequently storing it away safely instead of releasing it into the atmosphere. Carbon Sequestration – Carbon sequestration is a term that describes processes that remove carbon from the atmosphere. • Seeding oceans with iron • Solar shades • Geoengineering Seeding Oceans With Iron • It is motivated by evidence that seeding the oceans with iron will increase phytoplankton populations, and thereby draw more carbon dioxide from the atmosphere. Solar Shades • Some scientists have suggested using aerosols and/or sulfate dust to alter the Earth's reflectivity by burning sulfur in the stratosphere, as an emergency measure to increase global dimming and thus stave off the effects of global warming. • It would, however, increase the environmental problem of acid rain and drought. Governmental And Intergovernmental Action • Policies like: – Kyoto Protocol – Carbon emissions trading – Carbon tax Population Control • The population explosion is a fundamental factor that has led to global warming • Because of this, various organizations promote population control as a means for mitigating global warming. Proposed measures include improving access to family planning and reproductive health care and information, public education about the consequences of continued population growth. Ozone (O3) is a dangerous street level pollutant, contributing to photochemical smog. It is also a minor greenhouse gas, contributing to climate change. The main reason people are aware of its existence is through its beneficial effects in the stratospheric ‘ozone layer’ 5 to 30 miles up, which provides an important protection for life on Earth from the dangerous effects of the sun’s radiation, by absorbing biologically damaging ultraviolet sunlight (UV-B). The hole in the ozone layer was first noticed over the British Antarctic Survey station Halley, Antarctica10. Today, up to 60% of the total overhead amount of ozone is depleted during the Antarctic spring. In the Arctic Polar Regions a similar but smaller hole has appeared in 6 out of the last 9 years. Increases in surface UV-B radiation have been observed in association with local decreases in stratospheric ozone, from both ground-based and satellite-borne instruments. A dobson unit is the most basic measure used in ozone research.One Dobson Unit (DU) is defined to be 0.01 mm thickness at STP (standard temperature and pressure). Ozone layer thickness is expressed in terms of Dobson units, which measure what its physical thickness would be if compressed in the Earth's atmosphere. Ozone-depleting compounds are a group of chemicals called halocarbons that can contain the elements chlorine, fluorine, bromine, carbon, and hydrogen. As early as 1974 an article in Nature11 had shown that compounds being added to the Earth’s atmosphere were destroying the ozone layer. One group of halocarbons called chlorofluorocarbons (CFCs) invented in 1928 found use in aerosols, foams, refrigeration, air conditioners, cleaning of electronic components, and as a solvent. Another group (halons) was used in fire extinguishers. Once released, halocarbons are long-lived and stable chemicals that rise up and persist in the stratosphere for many years, where they break down ozone. Policy response on Ozone depletion The UN started to address the problem in the 1970s, resulting in the 1987 Montreal Protocol on Substances that Deplete the Ozone Layer. The Protocol aims to reduce and eventually eliminate the emissions of man-made ozone depleting substances, by stopping their production and use, and has been modified or strengthened five times so far by amendments. As more ‘culprit’ depleting substances are identified, the scope of the Protocol has been expanded. The UN Secretary General said in September 2000 that without the Protocol, the levels of ozone-damaging substances would have been five times higher than they are today, but developing countries are yet to phase out CFC emissions to meet the 2010 deadline imposed by the Montreal Protocol. There are also reports of a black market in CFCs. According to a recent UNEP news release, scientists predict that the ozone layer will fully recover some time in the 21st century – ‘but only if the Protocol continues to be vigorously enforced’. Acid Rain Introduction to acid rain Normal rain water is always slightly acidic because CO2 present in atmosphere. get dissolved in it form carbonic acid. Normal acidity of rain water is 5.6 H2O (l) + CO2 (g) H2CO3 (aq) Because of SO2 & NO2 gases as pollutants in atmosphere. The pH of rain is further lowered to as 2.4 & this type of Precipitation is called as ACID RAIN. Acid rain is combination of H2SO4, HNO3 and HCl is third History Since industrial revolution, emissions of SO2 & NO2 in atmosphere have increased. In 1852 ROBERT ANGUS SMITH was first to show relation b/w acid rain & atmosphere pollution in Manchester (England) Term acid rain was generated by SMITH in 1972. Problem of acid rain has not only increased with population & industrial growth but has become widespread. Acid Rain Formation Emissions of sulfur dioxide and nitrogen oxides react with water vapor in the atmosphere to create sulfuric and nitric acids. Causes Of Acid Rain NATURAL CAUSES:Volcanic emissions. Biological processes. Lightning. ANTHROPOGENIC CAUSES:Factories (industrialization) Motor vehicles, automobile exhaust. Coal based power plants. Domestic fires. Smelters. Measurement of acid rain Acid rain is measured through pH tests that determine the concentration of hydrogen ions in a liter of fluid. The pH (potential for hydrogen) scale is used to measure acidity or alkalinity. It runs from 0 to 14. (The greater the concentration of hydrogen ions and the lower the pH number, the more acidic a substance is; the lower the concentration of hydrogen ions and the higher the pH number, the more alkaline—or basic—a substance is.) So a pH greater than 7 indicates an alkaline substance while a pH less than 7 indicates an acidic substance Chemical Processes Involved In acid rain Formation Of Sulphuric Acid S + O2 SO2 + 1/2O2 + H2O SO2 H2SO4 Reaction Involving Formation Of Nitric Acid NO + O3 NO2 + O3 NO3 + NO2 N2O5 + H2O NO2+O2 NO3+O2 N2O5 2HNO3 Adverse Effect Of Acid Rain Plants Effects plants and trees. Causes yellowing leaf tissue (chlorosis). of Direct effect on plant growth due to toxification of soil It takes away soil nutrients causing stunted growth. Block stomatal pores of leaves. Electron transport system, biochemical reactions dominated by pH are effected. Degradation chlorophyll. of plant b. Soil Acid rain damages soil biology and chemistry Microbes not able to tolerate low pH and die Upper fertile layer of soil is affect as essential nutrients are leached away from soil a. Surface Water And Aquatic Animals Acid rain causes lower pH & high aluminum conc. in surface water that causes damages to fish and aquatic animals. Biodiversity of water body is reduced. Lakes, rivers are fragile ecosystems where each species depend on other to survive ,if one disappears other too disappears. c. Human Health Aerosol mist of sulfuric acid has very serious respiratory effects. Acidification play havoc with human nervous system ,respiratory system and digestive system. e. Effect On Buildings Causes extensive damage to buildings, structural materials of marble ,limestone, slate etc. CaCO3+H2SO4 CaSO4+H2O+CO2 In Greece and Italy invaluable stone statues have been partially dissolved by acid rain. Taj Mahal in Agra is also suffering due to acid fumes from Mathura refinery. Deterioration of Taj Mahal Taj, the seventh wonder of world getting deteriorated because of emissions of Mathura oil refinery which lies 40 km away from Taj The oil refinery emits 25-30 tones of SO2 daily in spite of using low sulphur fuels. Control measures • Clean combustion technologies • Using pollution control equipments • Replacement of coal by natural gas or renewable energy resources • Liming of lakes and soils • Formulate the policy framework for reduction of sulfur dioxide and other acid rain causing gas emissions. • Support a set of subproject that promote cleaner production, reduce acid rain and air pollution, improve the environment. “Soft” (Bituminous) Coal This is coal that has a low sulfur content. Liming The process of adding a lime or calcium hydroxide (a base) to acidified lakes. Use Energy Sources that Don’t Produce Nitrogen or Sulfur Oxides Health Effects of Air Pollution Air Pollution • The Clean Air Act requires EPA to set National Ambient Air Quality Standards (NAAQS) for six common air pollutants (also known as “criteria air pollutants"). • These pollutants are found all over the U.S., some from natural sources and some from man-made sources. • They can harm your health and the environment, and cause property damage. Criteria Air Pollutants • • • • • • carbon monoxide lead ground-level ozone particulate matter nitrogen dioxide sulfur dioxide Carbon Monoxide • Incomplete oxidation of carbon results in the production of carbon monoxide. – Natural CO formation occurs from photochemical reactions in the troposphere, volcanoes, forest fires, etc. • Breathing air with a high concentration of CO reduces the amount of oxygen that can be transported in the blood stream to critical organs like the heart and brain. • At very high levels, which are possible indoors or in other enclosed environments, CO can cause dizziness, confusion, unconsciousness and death. Lead • As a result of EPA's regulatory efforts including the removal of lead from motor vehicle gasoline, levels of lead in the air decreased by 98 percent between 1980 and 2014. Lead • Once taken into the body, lead distributes throughout the body in the blood and is accumulated in the bones. • Depending on the level of exposure, lead can adversely affect the nervous system, kidney function, immune system, reproductive and developmental systems and the cardiovascular system. • Lead exposure also affects the oxygen carrying capacity of the blood. Ozone • Breathing ozone can trigger a variety of health problems, particularly for children, the elderly, and people of all ages who have lung diseases such as asthma. • Ground level ozone can also have harmful effects on sensitive vegetation and ecosystems. Ozone Particular Matter EPA groups particle pollution into two categories: • "Inhalable coarse particles," such as those found near roadways and dusty industries, are larger than 2.5 micrometers and smaller than 10 micrometers in diameter. • "Fine particles," such as those found in smoke and haze, are 2.5 micrometers in diameter and smaller. These particles can be directly emitted from sources such as forest fires, or they can form when gases emitted from power plants, industries and automobiles react in the air. Particulate Matter Particulate Matter Particulate matter contains microscopic solids or liquid droplets that are so small that they can be inhaled and cause serious health problems. Nitrous Oxides • Breathing air with a high concentration of NO2 can irritate airways in the human respiratory system. – Such exposures over short periods can aggravate respiratory diseases, particularly asthma, leading to respiratory symptoms (such as coughing, wheezing or difficulty breathing), hospital admissions and visits to emergency rooms. – Longer exposures to elevated concentrations of NO2 may contribute to the development of asthma and potentially increase susceptibility to respiratory infections. People with asthma, as well as children and the elderly are generally at greater risk for the health effects of NO2. – NO2 along with other NOx reacts with other chemicals in the air to form both particulate matter and ozone. Both of these are also harmful when inhaled due to effects on the respiratory system. Sulfur Oxides • The largest source of SO2 in the atmosphere is the burning of fossil fuels by power plants and other industrial facilities. Smaller sources of SO2 emissions include: – industrial processes such as extracting metal from ore – natural sources such as volcanoes – and locomotives, ships and other vehicles and heavy equipment that burn fuel with a high sulfur content. Sulfur Oxides • At high concentrations, gaseous SO2 can harm trees and plants by damaging foliage and decreasing growth and can contribute to acid rain which can harm sensitive ecosystems. • Short-term exposures to SO2 can harm the human respiratory system and make breathing difficult. Children, the elderly, and those who suffer from asthma are particularly sensitive to effects of SO2.