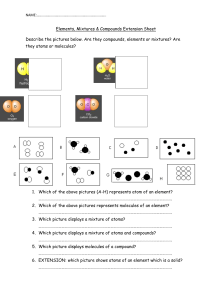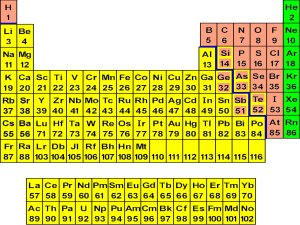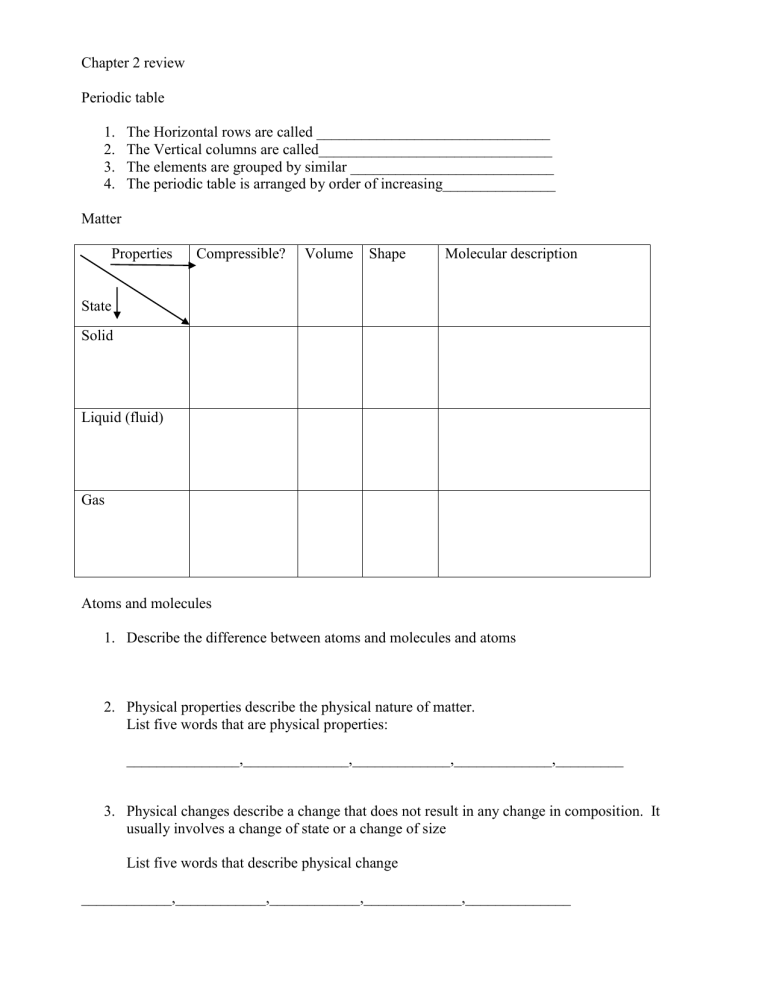
Chapter 2 review Periodic table 1. 2. 3. 4. The Horizontal rows are called _______________________________ The Vertical columns are called_______________________________ The elements are grouped by similar ___________________________ The periodic table is arranged by order of increasing_______________ Matter Properties Compressible? Volume Shape Molecular description State Solid Liquid (fluid) Gas Atoms and molecules 1. Describe the difference between atoms and molecules and atoms 2. Physical properties describe the physical nature of matter. List five words that are physical properties: _______________,______________,_____________,_____________,_________ 3. Physical changes describe a change that does not result in any change in composition. It usually involves a change of state or a change of size List five words that describe physical change ____________,____________,____________,_____________,______________ Changes of state are physical changes, write the appropriate word for the following descriptions; Solid to Liquid___________________ Solid to Gas___________________ Liquid to Solid___________________ Liquid to Gas__________________ Gas to Liquid____________________ Gas to Solid___________________ Physical properties can also be used to separate mixture into their substituent substances; Substances- Pure, either elements or compounds. Elements or atoms cannot be broken down further and still be able to retain the properties of the element. Compounds can be chemically broken down into their substituent elements. List four substances that are elements and four that are compounds elements_______________,_______________,______________,______________ compounds_______________,_______________,_____________,______________ Mixtures Homogeneous- A mixture that has the some composition throughout. List 4 homogeneous mixtures _____________,______________,______________,______________ Heterogeneous mixtures- A mixture that has distinct components, not the same throughout. List 4 heterogeneous mixtures _______________,______________,_______________,_______________ Sporadic hints 1. 2. 3. 4. If something has a formula or chemical name than it is a substance Dissolving is a physical process mixtures are separated by physical means, substances by chemical means( compound) There are five branches of chemistry; Analytical-_______________________ Inorganic________________________ Biochemistry_____________________ Organic__________________________ Physical__________________________ 5. Two feature that distinguish matter are mass and volume ( the density formula) 6. Pure substance, Buckminsterfullerene, Solution, Molecule, Atom, Alloy, Reaction, Graphite, and distillation are the terms for the matching. 7. Essay- In one experiment, magnesium metal is melted, in a second, it is burned, Classify the change in each experiment as a chemical or a physical change. Explain you reasoning. 8. Essay- explain the difference between a substance and a homogeneous mixture: