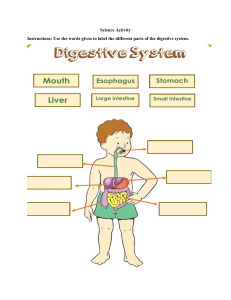
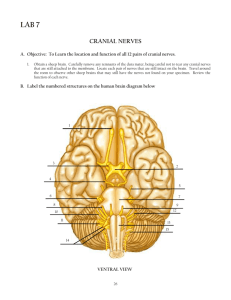
PRAC 5 BIOL 3510 Vertebrate Evolution MAMMALS - RODENTIA Aims: The aim of this prac is to continue to examine the evolutionary innovations of vertebrates, with an emphasis on mammals. We will focus on the digestive system but also continue to examine the respiratory and circulatory systems. In particular, we focus on the relationship between diet and digestive morphology. Learning outcomes: By the end of this practical you will be able to: ● Identify derived characters in extant mammals ● Relate characters to evolving complexities in vertebrates, particularly mammals ● Perform dissections to enable identification of mammalian anatomical features ● Collect and analyse morphometric data ● Evaluate findings relative to dietary adaptations within the Order Rodentia Mammals have a very fast pace of life. They have a high metabolic rate and need a lot of food to fire the furnace. They have a sophisticated lung that is supplied by a twin circulatory system that separates oxygenated from deoxygenated blood. The heart is necessarily four chambered with twin atria and ventricles each providing the pump for the two loops: one supplying the body (systemic) and the other supplying the lungs (pulmonary). Each loop necessarily varies in blood pressure, which must be high in the systemic system to fight gravity and low in the pulmonary system to preserve the delicate lung membrane integrity. Mammals are amniotes with internal fertilization. The young are suckled on milk produced by mammary glands. The skin is covered with fur to varying degrees. Sweat glands assist in thermoregulation. They have an enlarged neocortex and 3 middle ear bones. Teeth, which have a unique enamel coating, are replaced once or not at all rather than continuously. TASK 1 – Getting to know your alimentary canal Provide the function of each of the following organs: Stomach = Temporarily store food. Contract and relax to mix and break down food. Oesophagus = The primary function of your esophagus is to carry food and liquid from your mouth to your stomach. Small intestine = It helps to further digest food coming from the stomach. It absorbs nutrients and water from food so they can be used by the body. Large intestine = The purpose of the large intestine is to absorb water and salts from the material that has not been digested as food and get rid of any waste products left over. Pancreas = During digestion, your pancreas makes pancreatic juices called enzymes. These enzymes break down sugars, fats, and starches. Liver = The liver regulates most chemical levels in the blood and excretes a product called bile. 1 Rectum = To store (and eventually lead to the removal) of feces. TASK 2 – Exploring diet and the anatomy of a digestive system 1. The lab rat that is commonly used in medical research has been bred specifically for laboratory testing. However, its lineage is originally the Brown rat (Rattus norvegicus). The brown rat is a cosmopolitan rat species possibly the most successful of all mammal species. Q2: What is the diet of the brown rat? Brown rats have an omnivorous diet, which typically consist of fruit, nuts, grains and vegetables. They will also eat human waste, insects, small mammals, bird eggs and nestlings. Q3: Is the brown rat a herbivore, granivore, omnivore or carnivore? The brown rat is an omnivore. 2. The literature suggests that there is a relationship between an animal’s diet and the size of various components of the digestive tract. Of particular interest is the relative size of the small intestine and the hindgut (large intestine + caecum) since animals that eat a lot of vegetation require hindgut fermentation to break down cellulose. So we are interested in the proportion of the total digestive tract is made up of the hindgut. Wang et al 2003 (Digestive tract morphology and food habits in six species of rodents. Folia Zool. 52(1): 51-55 – see iLearn.) examined this relationship in six species of rodents. Plot the findings of their study and form a hypothesis about the brown rat’s digestive tract morphology. The data you will require is in the EXCEL spreadsheet labeled: Practical 5 - Wang datasheet on iLearn. Percentage taken up by the hindgut Hypothesis: The hindgut in the omnivore species will be smaller and less complex than in the herbivores. 60 50 40 30 20 10 0 Species type 2 Prediction: The species that will eats only plants will have a larger hidgut compared to the species who are omnivores. TASK 3 – Dissection of a rodent Subphylum: Vertebrata Class: Mammalia Subclass: Theria Infraclass: Eutheria (placental mammals) Order: Rodentia Genus and species: Rattus norvegicus External Features Refer to the external features of the brown rat video. Take a screenshot and identify the following: ● eyes, ears, mouth, teeth, hair, whiskers, feet with claws, anus, tail, male/female genitalia, nipples. 3 Internal features Method for dissection: Although you will be watching a video of the dissection, the following is a practical guide to the dissection methods. Note: To avoid damage to internal organs when cutting through the abdominal wall, keep the points of the scissors up and away from the internal system. 1) Use blunt forceps to lift the skin at the midline approximately halfway down the abdomen and make a small incision 2) Using a pair of fine scissors carefully slice away the fur to reveal the skin 3) With the scissors, make an incision towards the head and then towards the tail to expose viscera 4) Make further incisions from the opening along each limb. 5) Peal back the fur to reveal the body wall. 6) Using a pair of fine scissors cut through the body wall making sure it is held at tension with a pair of forceps. Make sure when you cut you keep the head of the scissors pointing up or parallel to the rat surface. This insures you do not cut through an organ. 7) Pin out the skin and body wall, but not too tightly. Before continuing, take a screenshot of the dissected rat and label it as we go 4 Digestive system Watch the video of the dissection where we examine the internal organs. As you view the opened rat, you will see the spleen, lying beside the liver (both dark red organs) just below the thoracic cavity; by gently laying out the intestine we reveal the mesenteric vessels. Note the hepatic portal vein carrying blood from the intestine to the liver. Based on the video, label the following in the diagram below: ● Submaxillary salivary gland, Trachea, Larynx, Thyroid, Thymus, Left and right atrium, ventricles, left and right lung, diaphragm, Liver, small intestine, Stomach, Spleen, Large intestine, Cecum. 5 TASK 4 – Removal of innards In the dissection video, we remove the digestive system and perform some measurements. The following is a brief description of the general methodology: Carefully, using forceps only, pare away the connective (mesenteric) tissue from the intestinal tract to rectum. Ensure that the digestive tract organs are separated from the associated fat bodies and mesentery. Cut through the oesophagus but do not cut the end of the rectum. The large intestine extends beyond the caecum while the small intestine precedes the caecum. Extend the digestive tract out to its full length and measure (to the nearest mm); 1. stomach 2. small intestine 3. caecum and 4. large intestine. We measure each at least three times in three different places to capture some of the variability and record the average in table below. Image 2: After we have extracted the gastrointestinal tract, take a screenshot of the components listed below and note the length of each component in the table below Note length and width of each organ in the table below. Region Stomach Small intestine Large intestine Caecum 5 72 20 6 1.6 0.465 0.7 2 Length (cm) Width (cm) 6 TASK 5: Variation in rodent digestive systems Add the data from the brown rat to the data collected by Wang et al. 2003, add it to the graph you made above and paste the new graph here. Compare and contrast your results with those outlined in Wang et al 2003. Where does the brown rat sit in comparison with these other species? Did the result support your prediction in Task 2? Brown Rat Species Type Phodopus robovskii Meriones unguiculatus Cricetulus barabensis Cricetulus triton Spermophilus daurica Microtus brandti 0 10 20 30 40 50 60 Percentage taken up by the hindgut The brown rat sits lower than all the species in the list. This is because the other species consume a lot more plant material than the brown rat thus the need for more complex digestive system. This is consistent with our prediction because it shows that those animals who eat more plant material have a longer hindgut due to the need to break down cellulose. TASK 6 – Circulatory System Watch the video examining the brown rats’ circulatory system. In order to see the heart, the associated blood vessels and the lungs, we need to carefully cut though the ribs. Firstly, we will remove the diaphragm, which is a thin membrane at the base of the ribs enclosing the heart cavity. Then we cut through the ribs on both sides and remove the rib cage completely. The circulatory network of the pelvic region will have been exposed with removal of the digestive tract. 7 Q5: Examine the circulatory system consisting of the heart, veins and arteries. Take screenshot of the dissection and label as many components as you can find using the following diagrams as a guide. 8 Q6: Describe how blood circulates through the heart and lungs in mammals and how this differs to amphibians and bony fish. The diagram below may help. Blood is pumped from veins of the systemic circuit into the right atrium of the heart, then into the right ventricle. Blood then enters the pulmonary circuit and is oxygenated by the lungs. From the pulmonary circuit, blood re-enters the heart through the left atrium. From the left ventricle, blood re-enters the systemic circuit through the aorta and is distributed to the rest of the body. In bony fish, oxygen enters the fish through the gills. The single Atrium and Ventricle heart then pumps it to the rest of the body. The same for the amphibians. 9 Prac 7 Biol 3510 Vertebrate Evolution Teeth & Bones Learning outcomes: by the end of this practical you will be able to Recognise the various mammalian teeth and relate morphology to diet Provide the dental formulae for any given mammal jaw Identify the components of the axial skeleton and the appendicular skeleton. PART A – EVOLUTION OF MAMMALIAN TEETH Aims of Part A: Teeth are intimately linked to diet and have evolved over evolutionary time to enable animals to exploit particular dietary niches. Here we examine teeth in mammals. You will become familiar with tooth morphology, learn to identify different sorts of teeth and apply a dental formula to mammal skulls. We will also use a phylogenetic reconstruction to trace back the evolutionary origins of marsupial teeth. Background Jaws have an interesting evolutionary origin having evolved from the first two pharyngeal arches that support the gill slits in the chordates (the ancestors of the vertebrates) and the hyoid arch. The hyoid arch suspends the jaws from the brain case. They first appear in the jawed fishes, initially in Chondrichthyans, and are of dermal origin. The jaw likely functioned to increased respiratory efficiency (a buccal pump) and only later was it incorporated into feeding. Which of the three skull cranial regions does the jaw belong to? The skull belongs to the region known as the Dermatocranium. What are the other two regions and what role do they play? The splanchnocranium supports gills and offers attachment to respiratory muscles. It also contributes to the jaws and hyoid apparatus. Secondly, the Chondorocranium provides scaffolding and protection for the developing brain and the sensory organs. It seems likely that teeth have their origins as dermal denticles in the skin which ultimately migrated to the jaw where they took on a feeding function. Read about it here. Mammals are characterised by heterodont teeth. That is, the teeth of mammals are differentiated into several types, from the front of the mouth to back; incisors, canines, premolars and molars (Fig. 1). Each of these different types of teeth display many different morphological variations directly related to their function in feeding. This has allowed mammals to not only adopt a wide variety of diets, but also to become highly efficient at biting and chewing. Fig 1: Upper and lower jaws of the Thylacine (Thylacinus cynocephalus) showing the different kinds of teeth. One of the evolutionary trends observed amongst different mammal lineages is a change in the number of each tooth type through time. Some mammals have lost teeth (e.g. rodents lose premolars, canines and some incisors) while others have secondarily increased their number of teeth (e.g. Odontocet dolphins). As a result, there is no consistent tooth number found in mammals. The different numbers of teeth and types of teeth is represented using the dental formula. The dental formula records the number of incisors, canines, premolars and molars in the upper and lower jaw of mammals. For example: the dental formula for the Thylacine pictured above is: 4.1.3.4 3.1.3.4 Fig 2: Tribosphenic upper and lower molars of the Virginia Opossum (Didelphis virginiana) showing the pattern of occlusion. To facilitate systematic descriptions of mammal teeth, every cusp has a specific name (Fig. 3). Researchers keep this nomenclature standardised so analogous cusps have the same name in all mammalian orders. The ancestral mammalian tooth is the tribosphenic tooth. The occlusal (top) surface of this basic type of tooth has three cusps (that is three elevated points on the occlusal surface) that are connected by ridges. The premolars and molars of each group of modern mammals are all derived from this basic condition. During the evolution of tribosphenic mammals, there were numerous modifications of the basic tribosphenic molar. A common first modification was to evolve from the basic triangular shape to a square shape by the addition of a fourth cusp (quadritubercular; Fig 3). Fig 3: Quadritubercular upper (left) and lower (right) molars of the Long-nosed bandicoot (Perameles nasuta). From the basic tribosferic morphology, a variety of more specialised upper molar cusp morphologies evolved such as bunodont, secodont, selenodont, lophodont and the plagiaulacoid 3rd molar of Macropodid marsupials (Fig 4). Fig 4: Cheek-tooth form in various mammals. All specimens are upper right teeth with both the buccal view (left) and occulusal (right) view shown. A, secodont carnassial; B, secodont premolar (marsupial plagiaulacoid); C, bunodont molar; D, lophodont molar; E, selenodont molar (From Ungar 2010, fig. 1.4, p. 15). In addition to molar and premolar teeth, there are a variety of other substantial tooth adaptations in Australasian mammals. Australian herbivorous marsupials of the Order Diprotodontia have a single pair of greatly enlarged procumbent lower incisors (called diprotodonty; Fig 5). Fig 5: Skull of Common Brushtail Possum (Trichosurus vulpecula) showing procumbent lower incisors. Fig 6: Skull of the Marsupial Lion (Thylacoleo carnifex) with greatly enlarged blade-like premolars. Exercise 1 1. Choose a skull to examine on pedestal and note down the species and any other taxonomic information (Family, Order etc.) Phylum: Chordata Class: Mammalia Infraclass: Marsupialia Order: Diprotodontia Family: Macropodidae Subfamily: Macropodinae Genus: Setonix (Quokka) 2. Take a screenshot of the entire dental row (upper and lower) and paste them here. Determine and record the dental formula. Dental formula: 3/1, 1/0, 2/2, 4/4 3. Inspect the upper molars. Can you recognize any molar specialisation (Fig.4)? Take close-up screenshots of the upper molars and paste them here. The Quokka has flat molars. This is because they feed mostly on plant materials and these flat materials help grind down plant material for digestion. 4. Are any other teeth highly modified? If so how? No, they are not. 5. Based on the morphology of the teeth what do you think the animal eats? Plant material. The animal is a herbivore. Exercise 2 1. Determine the dental formulae for the following animals: Placental mammals: Deer – I 0/3, C 0/1, P 3/3, M 3/3 × 2 = 32 Dog – I 3/3, C 1/1, P 4/4 M 2/3 x2 = 42 Cat – I 3/3, C 1/1, P 3/2, M 1/1 x2 = 30 American marsupial: The Virginian opossum – I 5/4, C 1/1, P 3/3, M 4/4 x2 = 50 Exercise 3 Below is a simplified phylogenetic tree of Australian marsupial families (Fig 7). Assign dental formulae to the missing families. You might find additional information online. Then, using your knowledge of phylogenies, try to work out what the ancestral dental formula was for all of the nodes in the tree and estimate the ancestral dental formula. Hint: Marsupials have lost teeth over evolutionary time and the ancestors of the Australian marsupials came from America (think Virginia Opossum). That means at each node we need to keep the maximum number of teeth in any given position as we move back in time. Table 1 shows the list of potential specimens for each of the families. See also 3D scans on pedestal Fig 7: Evolutionary relationships of selected families of Australasian marsupials. * = no specimen Table 1: List of specimens available Family Species Common Dasyuridae Antechinus stuartii Brown Antechinus Dasyuridae Sarcophilus harrisii Tasmanian Devil †Thylacinidae Thylacine sp. Tasmanian Tiger Peramelidae Isodon obesulus Southern Brown Bandicoot Peramelidae Perameles naguta Long-nosed Bandicoot Phascolarctidae Phascolarctos cinereus Koala †Thylacoleonidae Thylacoleo carnifix Marsupial Lion Vombatidae Vombatus ursinus Common Wombat †Diprotodontidae Nototherium sp. Diprotodon Molar Casks Hypsiprymnodontidae See iLearn Rat kangaroos / bettongs Potoroidae Potorus tridactylus Long-nosed Potoroo Macropodidae Dendrolagus lumholtzi Lumholtz’s Tree-kangaroo Macropodidae Dorcopsis sp Greater Forest Wallaby (PNG) Teeth & Jaw Caste Macropodidae Dorcopsis hageni Greater Forest Wallaby (PNG) Macropodidae Setonix brachyurus Quokka Macropodidae Macropus giganteus Eastern Grey Kangaroo Macropodidae Macropus rufus Red Kangaroo Macropodidae Thylogale stigmatica Red-legged Pademelon Burramyidae See iLearn Pygmy Possums Didelphidae Didelphis virginiana Virginia Opossum (USA) Ornithorhynchidae Ornthorhynchus lanatinus Platypus Phalangeridae Phalanger musculatus Common Spotted Cuscus Phalangeridae Trichosurus vulpecula Common Brushtail Petauridae Petaurus breviceps Sugar Glider Pseudocheiridae Pseudocheirus peregrinus Common Ring Tail Felidae Panthera onca Jaguar Skull Canidae Canus familiaris Domestic Dog Cervidae Unknown Deer Bovidae Unknown Goat More placentals are available on pedestal PART B – BONES, BONES AND MORE BONES Aims of Part B: You cannot talk about vertebrates without mentioning bones. This part of the practical provides a brief examination of the complexity of the post-cranial skeleton. This includes the axial skeleton (vertebrae) and the appendicular skeleton (limbs). The complexity of both these structures came subsequent to the movement onto land, although the precursors to limbs are obviously seen in the lobe-finned fishes. The key innovations of both the axial and appendicular skeletal structures is directly related to an animal’s need to support its own weight, protect vital organs and support key muscle groups important for both movement and organ function. Fig 8. The major bones in the rat skeleton Part 1: Skeletal basics Limbs: 1. Using the diagram of a rat skeleton above (Fig 8) - Match the common names with the following bone names. Common names: Shoulder, Upper arm, Lower arm, Wrist, Hand, Fingers, shoulder blade. Bone names: Ulna, Phalanges, Pectoral girdle, Scapula, Radius, Carpals, Humerus, Coracoid, Metacarpals. Shoulder = Coracoid Upper arm = Humerus / Pectoral Girdle Lower arm= Ulna and Radius Wrist = Carpals Hand = Phalanges Fingers = Metacarpals Shoulder blade = Scapula 2. The rat exhibits the maximum number of regions (5) of the axial skeleton. What are their names? They are: 1. The skull 2. rib cage 3. hyoid bone 4. sternum 5. vertical column 3. To become familiar with the structure of vertebrae examine, use the following terms to label various parts of the vertebra below. ● ● ● ● ● ● ● ● ● ● ● ● Body Anterior tubercle Posterior tubercle Lamina Spinous process Vertebral canal (foramen) Inferior articular process Pedicle Transverse canal (foramen) Spinal nerve groove Transverse process Superior articular facet 4. Are there noticeable differences in the shape of the vertebrae in the different regions? Why? Part 2: Comparing limbs Using the skeletons provided in the resources on iLearn fill out the table below: Taxa Pectoral Pelvic Direction Elbow Sternum Wrist Leg Position Regions Fish Attached Forward Absent Absent Absent Lateral 2 Tetrapodomorph s - Tiktaalik Early tetrapods Acanthostega Frog Attache d Attache d Attache d Beneath Absent Present Absent Beneath 3 Attached Attache d Beneath Present Present Present Lateral 3 Attached Forward Present Present Present Lateral 3 Salamander Attached Beneath Present Present Present Beneath 4 Lizard Attached Forward Present Present Present Beneath 4 Bird Attached Beneath Present Present Present Lateral 4 Mammal Attached Attache d Attache d Attache d Attache d Attache d Forward Present Present Present Lateral 6 Traits: Pectoral = Attached or not to the axial skeleton (directly) Pelvic = Attached or not Direction = the direction the tip of fore leg/fin points at rest – forwards or back Elbow = present or absent Wrist = present or absent Sternum = present or absent Leg position = Fins/legs held lateral to or beneath the body Regions = How many differentiated regions of the axial skeleton does the particular taxa have. Using the information in this table describe the link between: 1. Regionalisation of vertebrae, the appearance of the pectoral and pelvic girdle, and the sternum. Animals on land all exhibit similar features. They also retain some of the features as animals that live in the seas. However, sea animals do not exhibit the same traits as one that live on land. 2. Weight-bearing and leg traits The more an organism weighs the more it will have its feet in a lateral position to support its body. 3. Leg/fin position and locomotion with respect to axis flexation (i.e. vertebrae flexes side to side or up and down) The legs/fins of these organisms all are attached in regions of the axial skeleton where locomotion is the easiest. This ensure that they can move with ease. Part 3: Turtles 1. Can the turtle vacate its shell? Examine the skeleton of the turtle in the resources on iLearn and determine if indeed a turtle could leave its shell if it wanted to. Justify your answer. No, it cannot. This is because the shell of a turtle is a part of its skeleton, so it is impossible to vacate its shell. 2. If it can’t move its ribs, how does a turtle breathe?! Turtles breathe through external openings located above their mouths. Air moves down these holes to the trachea. The trachea of a turtle is very flexible. This allows it move its head in and out of its shell. Part 4: Skeletons and locomotion Flight: Birds and bats have highly modified skeletal systems that have evolved through fusion and reduction of bones. Have a look at the skeletons of the two animals in the resources on iLearn. What are some of the skeletal features related to their ability to fly? Compare and contrast. Some features that allow flight are extraordinary light bones as well as having fewer overall bones than other organisms. In comparison, other animals have denser bones that have more fusion between them and hence are too heavy to lift themselves from the ground. Jumping: Frogs also have highly modified skeletal systems that have evolved through fusion and reduction of bones. Have a look at the frog skeletons in the resources on iLearn. What are some of the skeletal features related to their ability to jump? Frogs have elongated legs; these legs allow them to jump significant distances with little to no impact on them. Walking and running: There are 3 forms of cursorial locomotion that are exhibited by the following skeletons. 1. Name and define these three forms of cursorial locomotion and link them to the correct skeletons. Sheep Dog Wallaby 2. Looking at the skeletons of the animals used to illustrate the three forms of cursorial locomotion, what do you notice about the limb length? The skeletons of the three forms of cursorial locomotion all exhibit similar length and follow a similar structure. That is to say the hind legs, or legs, tend to be more bent . 3. How are the above types of locomotion related to the behavioural ecology of the organisms? All the animals live in environments that require them to be constantly moving, either from predators or to find new food. Hence, the need for this type of locomotion. Part 5: Convergent Evolution Limbs have been lost three times. 1. Can you think of which tetrapods have lost their limbs? An example of a tetrapod that has lost its limbs are snakes. 2. For what reasons can you suggest these limbs have been lost? Because snakes began to burrow for safety and legs were not suitable under the ground. Convergent evolution can cause headaches. It is very easy to think that animals fly has the same evolutionary origins as other organisms that fly. Likewise, one could be forgiven for thinking whales are more closely related to fish than a salamander. But we know better. 3. Examine the skeleton of a dolphin in the resources on iLearn and come up with a list of skeletal features that support the notion that whales are more closely related to Salamanders than they are to Fish. Whales have similar bone structures to salamander as opposed to fish. This means they are more closely related to salamanders than fish. Prac 8 Biol 3510 Vertebrate Evolution The Brain and Cranial Nerves Aim: To familiarise yourself with the general form and function of the vertebrate brain and cranial nerves. Learning outcomes: by the end of this practical you will be able to Identify the main brain lobes of the vertebrate brain and be familiar with their function Name the 10 main cranial nerves in vertebrates and have some idea of their function Differentiate between the cranial nerves of fish and sharks relative to terrestrial vertebrates Phylum Chordata Class Chondrichthyes (cartilaginous fishes - sharks, skates, rays, chimaeras) Subclass Elasmobranchii Order Rajiformes (guitarfishes & skates) FamilyRhinobatidae (guitarfishes) Species Aptychotrema rostrata (Eastern shovelnose ray) Chondrichthyes (sharks and rays) have a cartilaginous skeleton. Unlike bony fish they lack a swim bladder and instead rely on a large, oil-filled liver to help control buoyancy. Rather than scales, their skin is covered with dermal denticles from which teeth are also derived. Their teeth sit on top of their jaws rather than being embedded. They have paired fins connected by girdle bars, paired nares and a 4 chambered, linear heart similar to bony fish. The notochord is replaced by the vertebral column. They have 5-7 gill slits, some also have a spiracle through which they can pass water while at rest. The intestines contain a spiral valve similar to that seen in the lungfish. Sharks are osmoconformers so their osmotic concentration is similar to seawater and they use urea as a counterbalance. This enables them to absorb water across their gills. They excrete salt via a rectal gland. No other vertebrate lends itself more admirably to the dissection of the brain and the cranial nerves than Chondrichthyes. The brain and nerves are large, easy to dissect and trace because of the soft cartilaginous skeleton. However, it must be emphasised that careful dissection is essential if you are to avoid damage to the delicate tissues of the nervous system. They are easily cut, torn or crushed. Before we begin exploring the brain, watch the external features video and think about how rays compare with other vertebrates. Task 1: The Brain While we have done the dissection of the brain for you, it is important to understand the process. To view the brain, we must first remove the skin from the entire head using a sharp scalpel. The brain is exposed by shaving off thin layers of the cartilage covering it. It is important to be careful not to cut any nerves passing from it. By dissecting largely on one side, we can leave the other side for reference in case we accidentally damage anything. Watch the dissection video and answer the following questions. Q1. What skull layer do you need to cut through to get to the brain? You will need to cut through the Chondrocranium to get to the brain of the animal. Q2. How is this layer different to other vertebrates? It is made of cartilage, so it is easier to cut through than bone. Other vertebrates have bony skulls. Q3. What importance does this layer have in the Chondrichthyes other than protecting the brain? It helps the animal swim in the deep ocean. The brain is composed of three distinct parts, the forebrain, midbrain and hindbrain. Each section is generally associated with a different sense, sight, sound, or smell (olfaction). Forebrain (prosencephalon) The forebrain can be divided into two segments. 1) The telencephalon is composed of: • olfactory bulbs - paired extensions of the brain which lie in contact with the olfactory sacs, • olfactory tract - a stalk which carries many olfactory nerve fibres between the olfactory bulb and the cerebrum (rostral end of the brain), • cerebrum - is made up of a pair of olfactory lobes which receive the olfactory tract. 2) The diencephalon (posterior part of forebrain) is a depressed region just caudal to the cerebrum and looks like a connection between the cerebrum and the optic lobes. A delicate vascular membrane known as the choroid plexus covers the diencephalon. This membrane secretes cerebrospinal fluid. Midbrain (mesencephalon) The midbrain consists of two rounded optic lobes. Hindbrain (rhombencephalon) The hindbrain can be divided into two segments. 1) The cerebellum - a large mass lying caudal to, or partially covering the optic lobes. It is divided into four parts by faint transverse and longitudinal grooves. 2) The medulla oblongata - an elongated caudal part of the brain, which narrows to form the spinal cord. It is covered by the posterior part of the choroid plexus. Paste an image of the ray brain here and label it with the main features listed above. The image below may help you. A = dorsal view, B = ventral view Task 2 - Cranial Nerves Nerves are classified (sensory or motor) according to the information they carry, the direction they carry it (afferent or efferent), and the tissue they serve (visceral or somatic). Sensory or afferent sensations travel along nerve fibres to the central nervous system. Motor or efferent impulses travel away to peripheral effector organs. Cranial nerves have roots enclosed in the braincase. Cranial nerves may consist of sensory fibres or motor fibres, or both (mixed). Identify the cranial nerves, starting from the most anterior and working your way carefully backwards. There are 10 cranial nerves, numbered in an anterior to posterior direction. Identify the cranial nerves that can be seen in a dorsal view using the figures and tables supplied. These nerves will include nerves I, II, III, IV, V, VII, VIII, IX and X. Note that for V and VII, which each have three major branches, when looking in a dorsal view you will only see the ophthalmic branch (both V and VII have this branch), the hyomandibular (branch of VII), and possibly the palatine branch of VII. To see the other two branches of V (the maxillary and mandibular branches), and to see the maxillary / buccal branch of VII (which runs alongside the maxillary branch of V) and perhaps the palatine branch of VII, you will need to do the following: Remove the tissue from the underside of the eyeball, so that you can lift the eyeball up. Look from the lateral (side) view when the eyeball has been lifted up, and you should see the branches of V and VII listed above. I. Olfactory nerve (sensory) (which you should have already located) extends from the telencephalon to the olfactory sac. They are associated with the sense of smell. II. Optic nerve - (sensory) carries visual impulses from the retina to the diencephalon and optic lobes. In order to see this nerve, you must carefully cut around the eyeball so as to free it in the eye cup. Do not cut any of the eye muscles as these are all innervated and you will not find the nerves if you have removed the muscles. Once the eyeball is free to move you will be able to see the largish nerve at its base. This leads into the brain. III. Oculomotor nerve - (motor) this is a small nerve which conveys motor impulses to all of the extraocular muscles (except the superior oblique and the lateral rectus). It enters the orbit (eye cup) just caudal to the optic nerve and innervates several of the eye muscles. It is often best located by looking on the ventral surface of the eyeball. IV. Trochlear nerve - (motor and sensory) this is an even smaller nerve, entering the orbit above the optic nerve and innervating the superior oblique eye muscle. V. Trigeminal nerve - (sensory and motor) by contrast this is a very large nerve, consisting of several branches, most of which you will locate in the orbit. The first of these is the superficial ophthalmic, which runs along the dorsal cartilage of the orbit forwards to the skin of the rostrum. It is joined anterior to the orbit by the deep ophthalmic, which runs through the back of the orbit. The second branch of the trigeminal runs along the bottom of the orbit, the infraorbital and the third branch, the mandibular, enters the orbit with the infraorbital but dives deeply to innervate the jaw. VI. Abducens nerve - (motor) this is the smallest of the motor nerves innervating the eye muscles. It enters the orbit at the base of the external rectus muscle and courses along its ventral border. It is not seen frequently - a challenge for the keen student! VII. Facial nerve - another very large nerve leaving the brain in association with the trigeminal nerve (just behind it) and sharing several of the nerves with the trigeminal. It has fibres in the superficial ophthalmic and in the infraorbital. These branches are part of the lateralis system. The third branch is caudal to the orbit so your dissection must now move further posteriorly - it is the hyomandibular, which innervates the spiracle, hyoid arch muscles, floor of mouth and tongue and can be located in the muscle curving around the spiracle posteriorly. The hyomandibular branches just after it leaves the brain into the palatine and dives quite deeply beneath the inner ear. Another challenge! VIII. Auditory (Statoacoustic) nerve - (sensory) a short nerve coming from the inner ear capsule to the medulla. It is located immediately posterior to the 5th and 7th nerve roots. IX. Glossopharyngeal nerve - (sensory and motor) innervates the second branchial pouch and the third branchial bar. Your dissection should locate the origin of both this and the next nerve (vagus) at the brain and carefully trace them back into the muscle surrounding the gill pouches. X. Vagus nerve - this is the last and longest of the cranial nerves in elasmobranchs. [Nerves XI and XII are spinal nerves in elasmobranchs]. It originates on the caudal half of the medulla and enters the dorsal wall of the rostral cardinal sinus. You must cut through the dorsal constrictor muscles at the upper ends of the gill slits to open this sinus and find the nerve. The vagus nerve innervates the remainder of the gill pouches, in addition to sending a branch, the visceral, to innervate the viscera of the body cavity. There is also a lateral branch supplying the lateral line canals. Watch the cranial nerves video and label each of the cranial nerves in the image below. Note that nerve VI - Abducens is identified in the video but can’t be seen in the image below. Task 3 – RAY DIGESTIVE TRACT For comparison, we have dissected the digestive tract of a male and female eastern shovelnose ray. Watch the video and answer the following questions. What unusual feature is present in the rays’ the digestive system? The feature which makes the digestive system unique is due to the massive spiral valve they have a shorter digestive tract. What other animals have you encountered during the practical classes that share a similar system? Task 4 - PREDATOR - PREY SCENARIO A tiger shark, Galeocerdo cuvier has just detected a scent indicating food. Consider the behavioural responses of the shark to the prey and how these are interpreted within the nervous system. Refer to the information on cranial nerves in Task 2 above to address the following questions: a) Describe briefly how the smell from the sharks prey is relayed to the brain (i.e. which cranial nerve) and what part of the brain does the signal is sent. Under the snout are two nares, or nasal cavities. Each nare has two openings, one for water to enter and one for water to exit. The shark sucks or pulls the water into the nares to sniff out any evidence of prey. The nasal sacs are filled with sensory cells, which send signals to the shark's brain. b) The shark then gets close enough to use visual senses, and starts looking for the prey. Which cranial nerves are involved with taking in visual information and directing eye movement? The Optic Nerve. c) What part of the brain does the visual information get sent to? Visual information gets sent to the Glossopharyngael nerve. d) The shark is then close enough to detect the electric field of the prey. Which cranial nerves (and which branches of these) are relaying electroreception information to the brain? The electroreceptors. e) The shark then opens its jaws to capture the prey, and begins biting and tasting. Which cranial nerves (and which branches of these) are directing the jaw muscles to contract (work), and which are relaying information about taste and mouth / throat volume back to the brain? Facial nerves help convey the information. f) The shark then swallows the prey. What kind of messages would the nerve X be transmitting from stomach to brain? What is the name of nerve X? The vagus nerve g) What sort of commands would get sent from the brain to the stomach via nerve X? The shark has just eaten and it is time for digestion. h) What other organs does nerve X regulate? All organs that belong to the digestive tract. i) While all this is going on, the shark is still respiring using its gills. Which cranial nerves are involved in detecting what is going on in the gills, and which are telling the muscles of the gills to pump? The glossopharyngeal nerve is telling the gills pump as well as detecting what is happening around the gill. j) What would these nerves (question i) regulate in an aquatic mammal such as a seal? The areas around their respiratory system.