Atomic Structure Test: Atoms, Elements, and the Periodic Table
advertisement
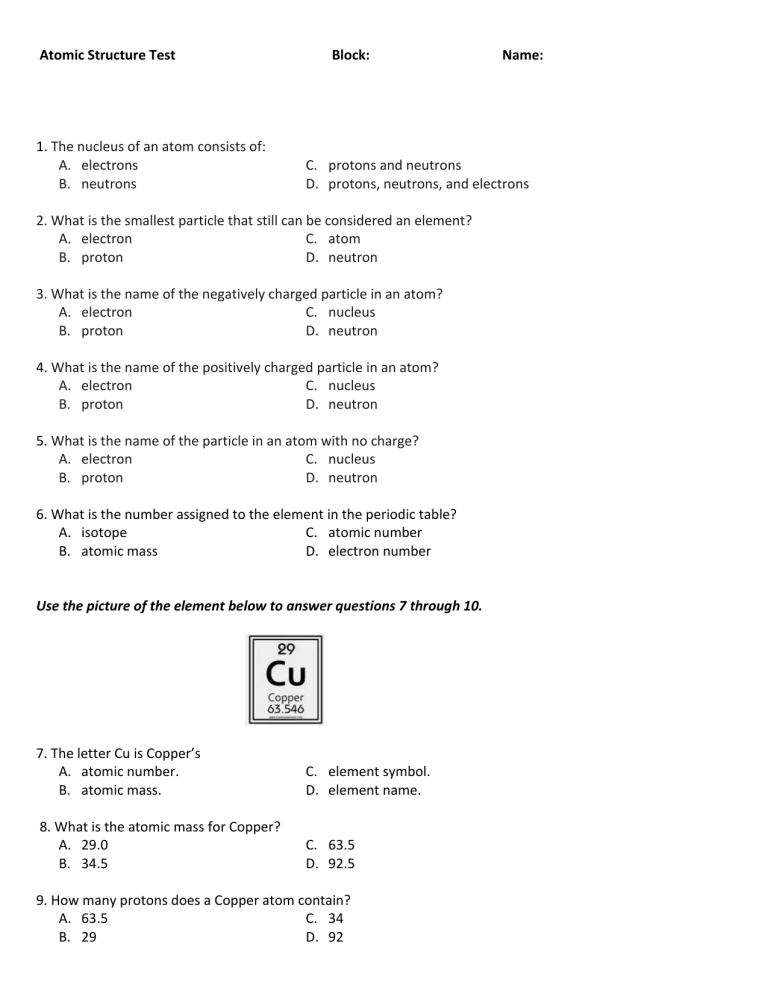
Atomic Structure Test 1. The nucleus of an atom consists of: A. electrons B. neutrons Block: Name: C. protons and neutrons D. protons, neutrons, and electrons 2. What is the smallest particle that still can be considered an element? A. electron C. atom B. proton D. neutron 3. What is the name of the negatively charged particle in an atom? A. electron C. nucleus B. proton D. neutron 4. What is the name of the positively charged particle in an atom? A. electron C. nucleus B. proton D. neutron 5. What is the name of the particle in an atom with no charge? A. electron C. nucleus B. proton D. neutron 6. What is the number assigned to the element in the periodic table? A. isotope C. atomic number B. atomic mass D. electron number Use the picture of the element below to answer questions 7 through 10. 7. The letter Cu is Copper’s A. atomic number. B. atomic mass. C. element symbol. D. element name. 8. What is the atomic mass for Copper? A. 29.0 B. 34.5 C. 63.5 D. 92.5 9. How many protons does a Copper atom contain? A. 63.5 C. 34 B. 29 D. 92 10. How many neutrons does a Copper atom contain? A. 63.5 B. 29 C. 34 D. 92 11. Fill in the chart Element Sodium Silicon Calcium Aluminum Lithium Beryllium Protons 11 14 13. What are the properties in Group 18? A. Air B. Metal C. Metalloids D. Noble Gases 14. What element is the same as AU (Gold)? A. Zn B. Cu C. Ni D. Fe 16. Which element is the less reacted? A. Pb B. Xe 14 20 13 3 12. What element is in Period 6: Group 7? A. Pd B. Nb C. Re D. Hg 15. Which element is the most reacted? A. Li B. Tc C. Cs D. H Neutrons 5 Atomic Mass 23 40 27 7 9 C. P D. Ne 17. How many protons does Titanium have? A. 11 B. 12 C. 23 D. 2 Use the picture of the Bohr atom below to answer questions 18-20. 18. How many electrons does this atom contain? A. 3 B. 2 C. 4 D. 9 19. What is the atomic mass of this atom? A. 3 B. 4 C. 7 D. 9 20. What is the atomic number of this atom? A. 3 B. 4 C. 7 D. 9 Bonus Questions 1. How do we find the neutron of an element? 2. What is the shell rule? 3. What element is this diagram below? 4.







