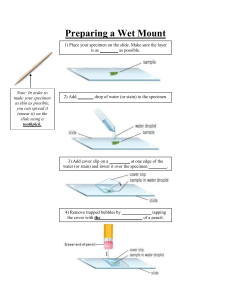
Methods of Culturing Microorganisms Specimen Collection Five basic techniques 1. Inoculate 2. Incubate 3. Isolation 4. Inspection 5. Identification Inoculation •Culture: the propagation of microorganisms with various media •Medium (pl. media): a nutrient used to grow microorganisms outside their natural habitat •Inoculation: the implantation of microorganisms into or onto culture media Incubation Incubator: media containing inoculants are placed in temperaturecontrolled chambers •Usual laboratory propagation temperatures fall between 20°C and 40°C •Atmospheric gases such as O2 and CO2 may be required for the growth of certain microbes •During incubation, microbes grow and multiply, producing visible growth in the media Isolation •Based on the concept that if an individual cell is separated from other cells on a nutrient surface, it will form a colony •Colony: a macroscopic cluster of cells appearing on a solid medium arising from the multiplication of a single cell Discussed in more detail later in notes •Requires the following - a medium with a firm surface - a Petri dish - inoculating tools Inspection Identification Isolation: A single visible colony represents a pure culture or single type of bacterium isolated from a mixed culture Isolation: Various Conditions of Cultures Pure Culture (a) One type of microbe Mixed Culture (b) More than one type of microbe Contaminated Culture (c) Unwanted microbe present Question • Can you isolate a colony using liquid culture? Three basic methods of isolating bacteria • Streak Plate • Loop Dilution • Spread Plate Isolation: Streak Plate Isolation: Loop Dilution Isolation: Spread Plate Media • Classified according to three properties – Physical state – Chemical composition – Functional types Physical State • 1. Liquid media (I.e. tryptic soy broth=TSB) • 2. Semi-solid media • 3. Solid media (I.e. tryptic soy agar=TSA) 17 Liquid media are water-based solutions that are generally termed broths, milks and infusions. Semi-solid media contain a low percentage (<1%) of agar, which can be used for motility testing. Solid media contain a high percent (1-5%) of agar, which enables the formation of discrete colonies. Chemical content • Synthetic media • Nonsynthetic or complex media Chemically defined media - media whose compositions are precisely chemically defined - contain pure organic and inorganic compounds that vary little from one source to another - • molecular content specified by an exact formula Minimal media - contain nothing more than a few essential compounds such as salts and amino acids - some contain a variety of defined organic and inorganic chemicals Complex media contain ingredients that are not chemically defined or pure (i.e. animal extracts). TSA + 5% sheep blood (Do we know the exact percentages of chemicals in the sheep blood?) 23 Functional types of growth media • Selective media • Differential media Comparison of Selective and Differential Media Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Mixed sample Mixed sample General-purpose nonselective medium (All species grow.) Selective medium (One species grows.) (a) General-purpose nondifferential medium (All species have a similar appearance.) (b) Differential medium (All 3 species grow but may show different reactions.) Selective media enables (selects) one type of bacteria to grow, while differential media allows bacteria to show different reactions (i.e. colony color) Selective media enables (selects) one type of bacteria to grow, while differential media allows bacteria to show different reactions (i.e. colony color) Differential media we will use in class Mannitol salt agar (MSA) contains 7.5% salt which selects for gram positive and also contains mannitol (carbohydrate) plus a pH indicator to indicate when mannitol has been fermented to an acid…media turns from pink to yellow Differential media we will use in class MacConkey agar contains bile salts and dyes that inhibit grampositive bacteria and hence selects for gram negative. Also turns dark color when lactose is fermented 28 Maintenance and Disposal of Cultures •Cultures and specimens constitute a potential hazard •Prompt disposal is required •Stock cultures represent a “living catalog” for study and experimentation •The American Type Culture Collection (ATCC) in Manassas, VA is the largest culture collection in the U.S. Microscopy Science relies on technology • The progress of microbiology and many other disciplines relies heavily on the introduction of new technologies • Technologies such as new types of media or microscopes lead to advances in microbiology • Without a microscope it would be impossible to characterize the morphology of bacteria. History of the Microscope • Development of the microscope originated in 1676 by Anton van Leeuwenhoek • Luckily, he was a skilled observer and artist and produced many fine illustrations of microbes (“animalcules”) • Unfortunately, he was not willing to share his microscopes or knowledge with other scientists until much later in life to help the progress of microbiology We measure specimens based on the metric system. We will be looking at bacteria ranging from 1 um to 10 um. 33 Principles of Light Microscopy •Magnification - objective lens: closest to the specimen, forms the initial image called the real image - ocular lens: forms the second image called the virtual image that will be received by the eye and converted to the retinal and visual image •Total magnification Power of Objective Usual power of ocular Total magnification 4x scanning objective 10x 40x 10x low power objective 10x 100x 40x high dry objective 10x 400x 100x oil immersion objective 10x 1000x Magnification is useless without good resolution. •Resolution (resolving power) - the capacity of an optical system to distinguish or separate two adjacent points or objects from one another - the human eye can resolve two objects that are no closer than 0.2 mm apart • The shorter the wavelength, the better the resolution (the ability to see 2 separate units) The Effect of Wavelength on Resolution (a) Low resolution (b) High resolution High resolution distinguishes magnified objects clearly Wavelength and Resolution • Is (a) or (b) resolved? • Which resolves images better, gamma rays or visible light? Properties of Light A, B, & C D • Light has the ability to a) reflect, b) transmit (pass through), c) absorb, d) refract and Resolution can be increased by using immersion oil. A compound microscope is typically used in teaching and research laboratories One objective lens can hold up to a dozen small lenses inside Optical microscopes • All have a maximum magnification of 1000x--2000X – – – – – – Bright-field Dark-field Phase-contrast Differential interference Fluorescent Confocal Types of Optical Microscopes Bright-field • Most commonly used in laboratories • Observe live or preserved stained specimens Types of Optical Microscopes Dark-field • Observe live unstained specimens • View an outline of the specimens Types of Optical Microscopes Phase-contrast • Observe live specimens • View internal cellular detail Fluorescent Microscopy • Fluorescence stain or dye • UV radiation causes emission of visible light from dye • Diagnostic tool Fluorescent staining on a fresh sample of cheek scrapings from the oral cavity. Fuzzy cells are cheek cells, distinct rods and cocci are bacteria. Red=dead, green=live Example of a confocal microscope Fluorescence or unstained specimen images are combined to form a three-dimensional image. Electron microscopy • Very high magnification (100,0001,000,000X) • Transmission electron microscope (TEM) – View internal structures of cells • Scanning electron microscope (SEM) – Three-dimensional images Transmission Electron Microscopy (TEM) Example of False-color Scanning Electron Microscopy (SEM) Comparison of optical and electron microscopes What does an electron microscope use to visualize specimens? Which type of microscope has better resolving power, brightfield or electron? In lab, we use Bright-Field Light Microscopy • We will perform the following light microscopy techniques: • Wet mounts – Wet mount and hanging drop methods • Smears – Spread, air-dry, heat fix • Simple Staining – Cheek cells with methylene blue (BI231) • Differential Staining – Gram stain – Acid-fast (time permitting) • Special Staining – Capsule stain – Flagellar stain (prepared slides since this is a difficult technique) – Endospore stain (time permitting) Wet mounts • We will use the hanging drop technique to investigate bacterial motility: – Advantages of the hanging drop: • Easy to prepare • Bacteria are live so we can see motility –Disadvantages of the hanging drop: •Requires special depression slide •Difficult to visualize since microbes are not stained •Bacteria are live and therefore slides must be disinfected Smears • Performing quality smears takes practice. There are many steps in the preparation of a smear that must be performed correctly. (Think of Goldilocks that wants her porridge “just right”) • 1. Smear bacteria onto slide from broth or agar • Too thick and you can’t see through specimen, too thin and you can’t find any bacteria • 2. Air dry • Again, you do not want too large a smear or it will take a long time for the smear to completely air dry. • 3. Heat Fix (see next slide) Smears (cont.) • 3. Heat Fix • Pass slide through flame quickly 3-4 times • Heat fix too little and organisms may wash off slide • Heat fix too much and organisms may be distorted • Heat fixation: – 1. kills organisms – 2. adheres specimen to slide – 3. promotes stainability of specimen Stains • Positive stains – Dye binds to the specimen • Negative stains – Dye does not bind to the specimen, but rather around the specimen. Which type of stain do we usually use? Why do positive stains bind to the specimen? What makes a specimen negatively charged? Positive stains are basic dyes (positive charge) that bind negative charge cells, and negative stains are acidic dyes (negative charge) that bind the background. Molecular structure of methylene blue (a positive stain attracted to ?) 60 Positive stains Simple stain – One dye • Ex. Methylene blue, crystal violet • In BI231, you applied one stain (methylene blue) to visualize your cheek (buccal) cells. Positive stains Differential stain – Two-different colored dyes • Ex. Gram stain • Allow us to differentiate between types of bacteria. – Gram Stain • Gram-positive vs. Gram-negative • See next slide – Acid-Fast Stain • Used to identify acid-fast bacteria with a waxy, lipid cell wall. • Detects Mycobacteria like M. tuberculosis and M. leprosae • Most of the bacteria in our lab in AcidFast negative Positive Stains: Gram Stain • Most frequently used stain • Dictates which type of antibiotic treatment • Gram-Positive – – – – Purple Penicillin Thick Peptidoglycan 4 P’s (Positive, Purple, Penicillin, Peptidoglycan) – 1 plasma membrane • Gram-Negative – – – – Red Streptomycin Thin peptidoglycan 2 plasma membranes 63 Positive stains Special stain – Emphasize certain cell parts • Ex. Capsule stain Special Stains We use these special stains to identify special structures that some bacteria possess • Capsule Stain • Capsule does not stain so we stain the background • Can not heat-fix or capsule will be destroyed • Flagellar Stain • Very difficult stain, we will look at prepared slides • Endospore Stain • The endospore is difficult to stain since it is made of a thick, hard to penetrate spore wall. We will boil the stain to help it penetrate the spore coat.