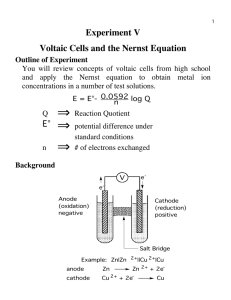
(a) Two bottles containing pent-1-ene, and hexane, require identification. Two reagents, bromine water, Br2, and acidified steam, H2O/H+, are available. Evaluate the possible use of both reagents to distinguish between the pent-1-ene and hexane. In your answer you should include: ● ● ● ● A description of the type of reactions that would occur Any conditions that would be required Any observations that would be made Equations showing the structural formulae of the organic reactant(s) and product(s) _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ _______________________________________________________________________________________________ (a) Pent-1-ene is an alkene that will readily react with both Br2 and H2O/H+. No extra conditions are required for these reactions to work. Alkenes when they react undergo addition reactions by breaking the double bond and freeing up space for new substances to be added. When pent-1-ene reacts with Br2 it forms 1,2-dibromopentane by breaking the double bond and adding 2 bromine atoms to the new space. During this reaction the orange/brown bromine water will be decolourised showing the reaction has taken place. Pent-1-ene will also react with the H2O/H+ to form an alcohol. This reaction forms two different alcohols, pentan-1-ol and pentan-2-ol. This is again an addition reaction as the double bond in the alkene is being broken and two new groups are being added in its place. This reaction will not show any colour changes as the chemicals are all colourless to start with and finish as colourless. Hexane is an alkane that will not readily react with the chemicals like an alkene. Akanes will undergo substitution reactions by swapping the groups for something else. For hexane to react with Br2, uv light and heat are required to encourage the reaction. When hexane reacts with Br2 one hydrogen is substituted with a Br forming bromohexane. This causes the orange/brown bromine water to decolourise becoming colourless. Hexane will not react with H2O/H+ so no observations will happen. (b) Addition reactions happen when we break a double bond and add 2 new groups to the carbons. This happens as when the double bond is broken it frees up two bonding sites, one of each of the carbons in the double bond. Polymerisation is the formation of a polymer molecule. This happens as a non-specific amount of monomers are added together to form a really long chained molecule. (Permanganate has been swapped for steam + acid)