General Physics 1: Unit Conversion & Scientific Notation
advertisement

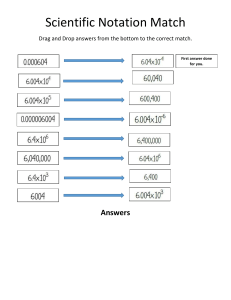
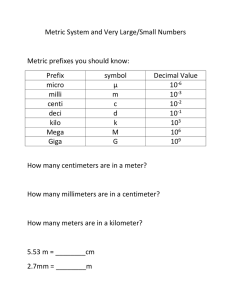
GENERAL PHYSICS 1 Subject Description: Mechanics of particles, rigid bodies, and fluids; waves; and heat and thermodynamics using the methods and concepts of algebra, geometry, trigonometry, graphical analysis, and basic calculus. UNIT CONVERSION AND SCIENTIFIC NOTATION At the end of the lesson, you are expected to: 1. Solve measurement problems involving conversion of units, expression of measurements in scientific notation. LEARNIN G OBJECTIV E What is Physical Quantity? A characteristic or property of an object that can be measured or calculated from other measurements. What is Unit? Reference standard to define the magnitude of a physical quantity. Significant Figures Determine the number of significant figures of the values given below: 1. 2. 3. 4. 5. 0.000982 = _____ 125.0010 = ____ 9.81 = ____ 100.90 = ____ 150 = _____ Rules in determining the number of significant figures: 1. All nonzero digits are significant. 256.78 985, 612 has 5 significant figures has 6 significant figures 2. All zeros between nonzero digits are significant. 8006 7.01 has 4 significant figures has 3 significant figures 3. All zeros to the left of the first nonzero digits are NOT significant; such zeroes merely indicate the position of the decimal point. 0.00001 has 1 significant figure has 2 significant figures 0.015 4. Zeros to the right of a decimal point in a number are significant. 0.900 7000 has 3 significant figures has 1 significant figure Rules for Mathematical Operations: 1. In adding or subtracting quantities, the least number of decimal places in any of the numbers being added or subtracted should also be the number of the decimal places in the answer. Ex: 3. 1918 m + 8.13 m + 0.156 m = 2. In multiplying or dividing quantities, the least number of significant figures in the input number should also be the number of significant figures in the answer. Ex: (10.30 mm) (0.50 mm) (2.01)= SCIENTIFIC NOTATION What is Scientific Notation? Scientific Notation, sometimes called as powers-of-10 notation, is a convenient and widely used method of expressing large and small numbers. It can be expressed as, 𝒏 N x 𝟏𝟎 , where: N = any number between 1 and 10 n = is the appropriate power of 10 Rules in expressing standard notation to scientific notation: 1. When the decimal point is moved from right to left, the result is positive exponent. Ex: 1269.4310 = 2. When the decimal point is moved left to right, the result is negative exponent. Ex: 0.0000003647 = Rules converting scientific notation back to standard notation: 1. Move the current decimal point according to the number of places based on the exponent. (+) positive exponent – move to the RIGHT Ex: 3.1416 x 104 = (-) negative exponent – move to the LEFT −3 Ex: 5.12 x 10 = Rules in Addition and Subtraction involving scientific notation 1. When two or more quantities are added or subtracted, make sure the exponents are the same. If not, choose one to adjust the decimal and exponent. Use LARS (Left Add, Right Subtract) 2. Add/subtract the number. Keep the exponent the same. Examples: 4 10 4 10 a. 3.41 x + 7.88 x = 6 3 b. 19.8 x 10 + 21.23 x 10 = 2 3 5 c. 56.3 x 10 + 0.45 x 10 + 3.06 x 10 = Rules in Multiplication and Division involving scientific notation 1. Powers of ten are added in multiplication. Ex: (2.4 x 102 )(10.85 x 103 ) = 1. Powers of ten are subtracted in division. 32.45×108 Ex: 2.50×105 = Practice Exercise: Express the following in scientific notation. 1. The amount of water surface area on the Earth is 140,000,000 miles2 . 2. The mass of a strand of hair is approximately 0.00000062 kg. 3. The length of the shortest wavelength of visible light (violet) is 0.0000004 m. SYSTEMS OF UNITS 1. Metric System (SI Unit) 2. English System Metric System (SI Unit) mks system – meter, kilogram and second cgs system –centimeter, gram, and second English System fps system – foot, pound and second TYPES OF PHYSICAL QUANTITIES Fundamental Quantities Derived Quantities Fundamental Quantities – are basic quantities that are independent of one another. SI Fundamental Quantities - length, mass, time, thermodynamic temperature, electric current, luminous intensity and amount of substance Fundamental/Base Units – units corresponding to the fundamental quantities. Derived Quantities – are combinations of fundamental quantities. Examples are speed, acceleration, density, work and energy. SI Fundamental Units Quantity Length Mass Time Temperature Electric Current Luminous Intensity Amount of Substance Unit meter kilogram second Kelvin Ampere Candela mole Symbol m kg s K A cd mol SI Prefixes SI Prefixes yotta zeta exa peta tera giga mega kilo hecto deka Symbol Multiplier Y Z E P T G M k h da 1024 1021 1018 1015 1012 109 106 103 102 101 SI Prefixes yocto zepto atto femto pico nano micro milli centi deci Symbol Multiplier y z a f p n µ m c d 10−24 10−21 10−18 10−15 10−12 10−9 10−6 10−3 10−2 10−1 UNIT CONVERSION CONVERSION FACTORS Length 1 ft = 12 in 1 yd = 3 ft 1 mi = 5280 ft = 1.609 km 1 in = 2.54 cm 1 m = 3.281 ft = 100 cm Volume 1 L = 1000 cm3 = 1000 ml 1 gal = 3.785 L = 4 qt = 231 in3 1 m3 = 1000 L CONVERSION FACTORS Time 1 min = 60 s 1 hr = 3600 s = 60 min 1 day = 24 hrs 1 yr = 365 days Force, Mass 1 lb = 4.448 N 1 slug = 14.59 kg 1 kg = 2.205 lb 1 kip= 1000 lb UNIT CONVERSION ILLUSTRATION: Convert the following: 1. 115 km = ___ ft 2. 600 µg = ___ g 3. 23 Terabytes = ___ bytes −8 4. 50 x 10 ns = ____ks 5. 0.700 nm/ms = ____ cm/min II. Solve the following measurement problems involving conversion of units. Express your answer in scientific notation. Answers should include three significant figures. 1. A government owned land will be set converted as a new wildlife refuge. Its dimensions are 15 × 1012 𝑚𝑒𝑡𝑒𝑟𝑠 by 10 × 106 𝑚𝑒𝑡𝑒𝑟𝑠. Find the area of the land in ℎ𝑒𝑐𝑡𝑎𝑟𝑒𝑠. II. Solve the following measurement problems involving conversion of units. Express your answer in scientific notation. Answers should include three significant figures. 2. One light-year (ly) is the distance travelled by light in a year. Convert one light-year to meters 8 using 3 x 10 m/s for the speed of light. 3. The rest energy E of an object with rest mass m is given by Einstein’s famous equation, E = 𝑚𝑐 2 , where c is the speed of light in vacuum. Find E for an electron for which m = 9.11 × 10−31 kg. The SI unit 2 2 for E is Joule = kg-𝑚 /𝑠 . Speed of light in a vacuum is 8 2.99792458 x 10 m/s .