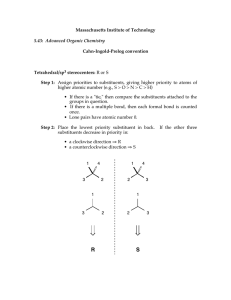
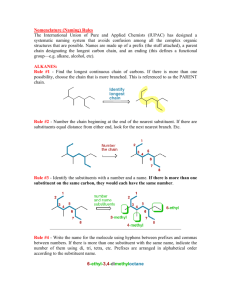
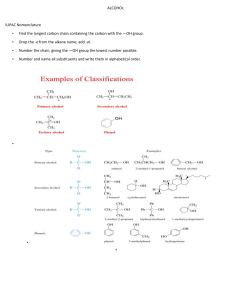
Hello and welcome to the Module 1 presentation. It is advised that before you watch this presentation you have read through the course notes in Module 1. The point of this presentation is to sort of summarize what you have read in module 1. Point out some of the key things that students struggle with and then work through some of the problems that you'll find at the end of module one in the course notes. Now we won't work through all of the examples because the idea is that you try a lot of these problems yourself based on what you have learned through reading the notes and watching this presentation, and then you self assess yourself by using the problem solutions that you also find in module 1. After self assessment, if you're finding that you're still struggling with the particular concept, please reach out to us using the class discussion board on Slate. Basically we started using IUPAC nomenclature in organic chemistry because when people started discovering different molecules, the naming of those molecules was a little bit random, so they were derived from sort of where the molecules came from, or they were named after a spouse or a child, and basically it was just sort of a random naming. Overtime organic chemist started to realize that this type of random naming system wasn't going to work and we needed something a little bit more consistent, so that organic chemists could understand each other better. The basic nomenclature starts with alkanes, which are the simplest organic molecules and they sort of make up the foundation of all of the parent names that we will encounter in this unit. So the simplest alkane is our one carbon alkane, alright and that is methane. And if we were to draw methane out as a 3D structure, it would have a tetrahedral shape. So if you remember back to 1st year when you talked about VSEPR theory, that's what we're referring to here. But don't worry, if you don't remember, we will actually review this in upcoming modules. Our two carbon alkane is referred to as ethane. Our three carbon alkane is referred to as propane. Our four carbon alkane is referred to as butane, and here I want to start talking a little bit about this bond line structure that we use so the bond line structure is shown here (butane bond line structure). And we draw a lot of organic molecules this way because it's just simpler. It's a faster way to draw organic molecules, and for those of you that aren't familiar with this way of drawing molecules, honestly, it just takes a little bit of practice and within a couple weeks of this course, most students find that they're just drawing molecules like this like they've been doing it all along. So the basics of this structure, Are that each vertex which here is represented by two and three. And each end, which is one in four. These each represent a carbon atom in the molecule. So because butane is a four carbon chain, we have four carbons. We also assume that because no other functional groups are drawn, that each of these carbons is then bonded to the appropriate number of hydrogens to saturate the carbons so we know carbons have four bonds, so each of these then has the appropriate number of hydrogens attached. Alright, the rest of the naming of alkanes, I think is pretty intuitive based on just knowing your shapes. So a five carbon chain is pentane so that you can think of pentagon and then hexane, hexagon, heptane, octane nonane and decane. Now you can find in textbooks that the naming of alkanes does go beyond ten, does go beyond decane. We're not going to do that in this course, so in this course you're only going to see up until decane. Now alkane chains are not just simply straight chain molecules, we often have what we refer to as substituents or branches. And the branch is basically the name of those is derived from the alkane chain. So for example, if a alkane branch has one carbon. Which we refer to as methane. The substituent is referred to as methyl, so we change that "ane" to "yl" to represent that it's an alkyl substituent. And the way that would sort of look on a straight chain is you can imagine you having one carbon substituent. Now all of these substituents also come with an abbreviation, so the one for methyl is just Me, the first two letters of the substituent, and so we could also see that substituent represented as Me. For the most part, in this course you generally won't see substituents but beyond four carbons, though, we might come across a pentyl sometimes, But generally we just stick with the methyl, ethyl, propyl and butyl. They all come with an abbreviation which is just the first two letters in the alkyl group name. And that first letter is always uppercase and the second letter is just lower case. That's sort of standard notation in organic chemistry for these substituents. Alright, so let's just walk through this example. This is the example that you find embedded in your course notes and what we ultimately want to do is we want to name this compound. So the first step in naming this compound is we want to find the longest continuous chain referred to as the parent chain. Now the parent chain may, or may not be shown as a straight horizontal line. So in this case we have two options that are effectively equivalent. So we have that parent chain (heptane). And we also have that parent chain (heptane). Both of them are seven carbons long. So we know that these are going to be referred to as heptane. OK, and either parent chain will give us the same ultimate name so it doesn't matter which one you decide to use the red one or the purple one. Now the next step is we have to number this parent chain so we have to number carbons 1 to 7. And the way that we number it is that the smaller number is used at the first point of difference, and this basically just means that you want to name the substituents in such a way that they have the lowest numbers possible. So in this case we're going to number the parent chain from right to left. OK, that gives our first point of difference or our first substituent the number 2. If we had numbered it from left to right, then our first substituent would have a number 4 which is higher. OK, now we want to name each of the substituents so we have a one carbon substituent. That would be our methyl or our Me. We have another one of those methyl groups. Here we have a two carbon substituent. So that is ethyl or Et. Now when we want to put those substituents into the name, we want to alphabetize them. So here we are only dealing with two ethyl and methyl so will alphabetize those. But now we need to indicate where on the parent chain those substituents are and then to simplify things if we have more than one of the same substituent instead of writing it out twice like methyl, methyl because we have two. We're actually just going to input the prefix "di" in front of the methyl. So here our ethyl. We will find on carbon 4. Oops, getting ahead of myself here. So we have four ethyl and then on carbons two and three. We can write 2,3-dimethyl instead of two methyl, three methyl. So this just simplifies things. And then we have to also include our parent name, which is heptane now the final step in naming our molecule is that we want to just sort of tidy it up a little bit, so 2 numbers that are adjacent to each other will be separated by a comma and numbers and names are separated by a dash, so our final name. Will look like 4-ethyl-2,3-dimethylheptane. And the dimethylheptane portion of the molecule is just put together as one word. There is no number that separates the dimethyl in the heptane. And now, so we're just going to sort of stick those together as as one word, there's no space there. Alright, so that's the final name of our molecule 4ethyl-2,3-dimethylheptane. Now a couple situations where you might encounter and you are not quite sure what to do. Is if you have two chains of equal length, competing to be the parent chain, how do you know which one to choose? So we have a couple of rules in place. This one sort of talks about how we want the chain with the greatest number of substituents, and so we're going to take this top chain here because it gives us four substituents rather than taking that other chain that is drawn, which would only give us three. So if you have two chains of equal length competing to be parent, you want to choose the chain with a greater number of substituents, alright, the other situation is when substituents it you know as you sort of walk in from the outer sides of the parent chain, they are equal distance from either end. So for example here the second carbon on either side both have a substituent. So how do we know how to number this chain? Well, basically because we have another substituent in the molecule, we want to give this substituent (methyl) the lowest number possible, and so in this case we're going to start numbering from right to left rather than left to right because we want to give that other substituent the lower #3 rather than the higher #4. Now, some side chains can exist in different forms, referred to as isomers. You probably heard about isomers in first year, so when we start getting into the propyl side chains. So the three carbon side chains. Those carbons can orient themselves in a different way and sort of. This just refers here, this line. This is how we're attached to the parent chain. So for example, We can have a propyl group that is attached to our parent chain that actually attaches at the second carbon rather than the first carbon. And so we have to name that side chain as well. And so we will you use these sort of common names shown here, and in this case this would be referred to as an isopropyl. We also have isobutyl, which is very similar except we have the extra carbon here. We then have two other isomers of butyl. We have sec-butyl, this is sort of this funny shape and then we have tert-butyl. Tert-butyl is most easily recognized because when it's a side chain it looks like a T so that's our tert-butyl or for short t-butyl came. This just takes practice. Just practice recognizing these ones and then it just kind of becomes second nature. Just like nomenclature in general. Just takes some practice there is this other one that we see from time to time refer to as neopentyl. It's similar to the tert-butyl but has that extra carbon there.