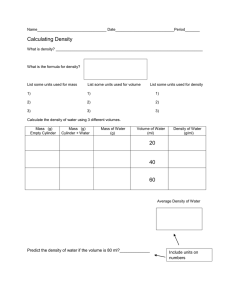
Physical Science Chapter 1 Review 1. In lab our 100 mL graduated cylinders have lines marking the 1’s place, therefore, they can be used to measure the uncertainty to the __________________ place. 2. The triple beam balances used in lab have lines marking the .1 place, therefore, they can be used to measure the uncertainty to the __________________ place. 3. Label the metric units that are used to measure each of the following: a. Mass ___________________________ b. Volume _________________________ c. Density __________________________ d. Distance _________________________ 4. In our demonstration with the box that was equal to 1000 cm3, how many mL were we able to perfectly poor into the container? ___________ This would mean that _____cm3 = _____mL 5. Define the following terms: a. Mass _________________________________________________________________ b. Volume _________________________________________________________________ c. Density _________________________________________________________________ 6. Answer the following with correct scientific notation a. 35,000 __________________________________ b. 0.00007 __________________________________ 7. Repeatedly achieving the same result is the definition of ________________________________ 8. Getting the exact number on an average of trials would be ______________________________ 9. If you wanted to identify an unknown silver metal, which of the following would be the most likely method of determining it? a. Mass the substance to see if it is a heavy metal b. Taste it c. Determine the volume so that you know if it takes up a lot of space d. Determine the density and compare it to a periodic table 10. Convert the following metric units: a. 5,000 mL = _________________L b. 0.075 m = _________________ cm When converting down, always move the decimal _____________, and when converting up, always move the decimal to the ______________. 11. Determine the error in each of the following problems: a. A student masses a piece of metal that is supposed to weigh 2.50 grams, but he gets a measurement of 2.45 grams. What is his error (how far off was he)? ______________________ What is his % error? ________________________ b. A student masses a piece of metal that is supposed to weight .25 grams, but she gets a measurement of .20 grams. What is her error (how far off was she)? ______________________ What is her % error? ________________________ What conclusion can you draw from the error the two students made in comparison to their % error? 12. Record how many significant figures are in each of the numbers below: a. 2,500 __________________ b. 0.03 __________________ c. 405 __________________ d. 0.0200 __________________ e. 121.00 __________________ 13. After massing a piece of copper metal three times, you find your measurements to be 12.95g, 12.93g, and 12.92g. What should you report as the mass of the object in grams? a. 12.93 b. 12.933 c. 30.19 d. 38.8 14. You fill a graduated cylinder to 15.5 mL. You then slide a zinc rod into the water, and the water rises to 26.5 mL. You should precisely report the volume of the zinc as ____________________mL. 15. Given the standard density table (see below), use it to determine the substance a student has that finds the mass to be 1,322.66 grams, and the volume to be 96.5 mL. Standard Density Table Wood .60 g/mL Aluminum 2.33 g/mL Iron 7.80 g/mL Copper 8.9 g/mL Mercury 13.7 g/mL 16. Using a meter stick, determine the width of this piece of paper in cm (width, not length): a. 21.65 cm b. 8.5 cm c. 22.7 cm d. 18.92 cm 17. When plotting a density graph, the mass would go on the _________________ axis, while the volume would go on the ___________________ axis. This way, the slope of the line (rise/run) would equal the correct units for density. 18. Would a beaker, flask or graduated cylinder be used to accurately measure the volume of a small piece of metal? ______________________________________ 19. After plotting the class data for mass and volume in the density lab. What type of line did you then have to draw? A line of ______________ ______________. Show a quick sketch of this type of line: 20. Use the graph on the following page to answer the questions below a. b. c. d. e. A slope of this line (rise/run) would have _____________________ for its units? (Hint: check the axis on the graph) Would the slope of this line indicate its mass, volume or density? _______________________ Use the graph to determine the slope of the line, and record your answer with the correct units here: _______________________________. Using the periodic table, determine what the substance might be _______________________. After verifying if your answer to “d” is correct with the teacher, determine the % error of the graph by comparing the slope value to the given value on the periodic table, show your work below, and record the % error with correct significant figures here: _____________________.