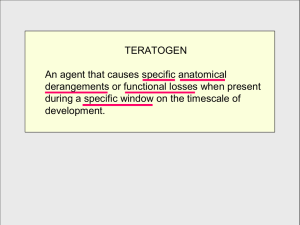
Teratogenesis https://www.youtube.com/watch?v=iN6uQYQ-hRU 30 min video on several teratogens- basic information Teratogenesis Learning objectives 1. Apply Wilson’s principles of teratology to understand what makes a teratogen. 2. Understand the different mechanisms by which teratogens exert their effects on the fetus. 3. Know why teratogens act in specific windows of susceptibility. 4. Appreciate that different teratogens have different mechanisms and often act through several different pathways. 5. Understand why commonly used substances such as alcohol and medications can also be teratogenic. The problem • Teratogens – agents that cause non-heritable birth defects. Teratogenesis • The environment not only gives signals for normal development but it also gives signals that can disrupt normal development. • 2-5% of infants are born with anatomical abnormalities which are caused by either genetics, the environment or a combination of the two. • Testing for teratogenicity is expensive and may not be transferable to humans. Teratogenesis Wilson’s principles of teratology 1. Susceptibility to the teratogenic effect of an agent depends on the genotype of the embryo, the genotype of the mother, and the ways in which their genotypes allow mother and fetus to interact with the adverse environmental factors. 2. There are critical periods of development when specific organ systems are most susceptible to being adversely affected by teratogenic agents. 3. Teratogenic agents act in specific ways on genes, cells and tissues in the developing organism to interfere with normal developmental events. Wilson’s principles of teratology 4. Several factors affect the ability of a teratogen to interfere with normal development. These include the nature of the agent itself, the route and degree of maternal exposure, the ability of the mother to detoxify or block the agent, the rate of transfer through the placenta, the rate of absorption by the embryo or fetus, and the genotype of the mother and her conceptus. 5. There are four major manifestations of abnormal development: death, malformation, growth retardation, and functional defects. 6. Manifestations of deviant development increase in frequency and degree as the teratogen dosage increases. Teratogenesis - definitions • Congenital anomaly – birth defect- can be structural or functional • Functional = intellectual (cognitive), emotional physiological • Structural = malformations, disruptions, deformations or dysplasias Teratogenesis-definitions • Malformation – structural birth defect which results from failure of tissue to initially form properly. • Disruption – Breakdown of a tissue that has initially formed properly. • Deformation- extrinsic mechanical forces on otherwise normal tissue. • Dysplasia – A lack of normal organization of cells in a tissue. Teratogenicity • Most teratogens produce structural defects only during certain critical periods of development. • Embryonic period – Conception to 8 weeks • Fetal period – remaining time in utero Dr. Ron Saulnier HSS 4102 Fall 2020 • Rare to have congenital anomalies before 3 weeks because teratogens damage either too many cells and it dies or too few cells and it recovers (pluripotent cells). • Maximum susceptibility is between 3-8 weeks when organs start to form. Except for CNS which forms continuously until adolescence. Windows of susceptibility Types of teratogens Mechanical forces Teratogens 1. 2. 3. 4. 5. Thalidomide Mercury Alcohol Retinoic acid Other teratogenic substances Thalidomide History • Thalidomide is a drug that was developed in the 1950s by the West German pharmaceutical company Chemie Grünenthal GmbH. • It was originally intended as a sedative or tranquiliser, but was soon used for treating a wide range of other conditions, including colds, flu, nausea and morning sickness in pregnant women. • In the 1960s, two medical professionals; Dr Widukind Lenze and Dr. William McBride, observed an association between the use of thalidomide in expecting mothers and congenital malformations. Thalidomide • Doctors were slow to make this connection due to the wide range of changes to foetal development. Limbs, internal organs including the brain, eyesight and hearing could all be affected. • Another reason why it took so long to establish the link to thalidomide was that some of the damage caused by the drug was very similar to certain genetic conditions that affect the upper or lower limbs. • It did not show teratogenic effects in rodents. https://www.sciencemuseum.org.uk/objects-andstories/medicine/thalidomide#:~:text=Thalidomide%20is%20a%20drug%20that,morning%20sickness%20in%20pregnant%20women. Thalidomide – window of susceptibility Figure 5.1 During the early 1960s, doctors (primarily in Europe) began prescribing the drug thalidomide to pregnant women as an effective mild sedative and remedy for morning sickness. Thalidomide – window of susceptibility Thalidomide- Effects • Phocoemelia - a congenital deformity whereby the hands and feet are bound to the child’s trunk, absent or grossly underdeveloped; • Disfigurements of the ear; • Ocular abnormalities; • Facial palsies; • Internal organ damage; • Increase in the number of miscarriages • Congenital heart disease. • Caused phocomelia in over 7000 infants – could occur with one tablet. • Over 80% of children born to mothers who took thalidomide had limb defects. • Only effective as a teratogen between days 20-36 after conception. Thalidomide • Thalidomide is a very complex molecule, requiring metabolic breakdown to achieve activity and forming potentially over 100 byproducts. The major functions of these by-products are antiinflammatory or antiangiogenic. • Blood vessels permit paracrine factor pathways necessary for limb bud formation. • NO promotes angiogenesis and protects from the effects of thalidomide. Thalidomide • Metabolized into several potentially teratogenic or nonteratogenic metabolites. • Acts primarily through blocking angiogenesis. • Once smooth muscles cover the newly formed vessels, thalidomide has no effect. • Thalidomide also displays immunosuppressive activity. It inhibits release of tumor necrosis factor-alpha from monocytes, and modulates other cytokine action. Thalidomide Thalidomide Figure 5.3 Thalidomide causes limb malformations by interfering with blood vessel formation in limb buds Current uses for thalidomide • Thalidomide is still a valuable drug for cancer treatment. • Also used in the treatment of some AIDS related conditions, leprosy, multiple myeloma, and cancers, as well as Crohn's disease, HIV, and others. Thalidomide R vs S Thalidomide story Mercury poisoning https://www.medicalnewstoday.com/articles/320563.php 26 Teratogenic agents - Methylmercury Heavy metal teratogens: industrial mercury and Minamata disease • Release of mercury compounds caused neurological abnormalities in nearly 10% of the children born near the area of Minamata Bay in Japan in the 1950’s. • A industrial plant producing acetaldehyde was releasing mercuric sulfate into the bay which was metabolized by microbes into methylmercury. • Methylmercury concentrated in the shellfish. 27 Methylmercury • Cats were dying, crows fell from the sky, fish were floating and some people had trouble seeing, hearing and swallowing. • For pregnant women, mercury was selectively absorbed in regions of the developing cerebral cortex. • Mice given Hg during pregnancy had pups with small brains or eyes. 28 Methylmercury toxicity • During the third week of gestation, the human nervous system begins to form in the embryo. • Methylmercury has toxic effects on the nervous system during embryonic development. • Methylmercury readily crosses the placenta to the fetus, where deposition within the developing fetal brain can occur. • In the brain, methylmercury causes focal necrosis of neurons and destruction of glial cells and is toxic to the cerebral and cerebellar cortex. • In children, defects due to methylmercury can result in deficits in attention, behavior, cognition, and motor skills. 29 Minamata disease This video is part of the class notes Figure 5.4 Methylmercury and Minamata syndrome 1.Protein inhibition 2.Disruption of mitochondria function 3.Direct affect on ion exchange in a neuron 4.Disruption of neurotransmitters 5.Destruction of the structural framework of neurons Mercury during development • • • • Methylmercury is especially dangerous to developing babies. Highly toxic and can cross the placenta and the blood-brain barrier. Concentrated in the brain of the developing fetus. Children exposed to mercury may be born with symptoms resembling cerebral palsy, spasticity and other movement abnormalities, convulsions, visual problems and abnormal reflexes. • The brains of children who have died as a result of mercury poisoning show neuron loss in the cerebellum and throughout the cerebral cortex. • Mercury also appears to affect brain development by preventing neurons from finding their appropriate place in the brain. Mercury – mechanism of action • Mercury has a strong affinity for sulfur, and mercury's primary mode of toxic action in living organisms is thought to be the interference of enzyme function and protein synthesis by binding to sulfhydryl or thiol groups. Alcohol Alcohol as a teratogen • Probably the most devastating teratogen in humans • 1- in 750 births are affected in the US, may be as high as 1-100 in some regions. • Produces a syndrome of birth defects – FAS 34 Fetal alcohol syndrome Fetal alcohol syndrome 36 Fetal alcohol syndrome 37 Alcohol affected Normal Figure 5.6 Effects of alcohol on developing brains Alcohol • Also have intellectual defects and behavioural abnormalities. • Behavioural changes can exist in the absence of gross physical changes. • Effects depend on the dosage and the developmental stage at time of exposure. • No safe level of alcohol during pregnancy. • Mice have been used as a model for FAS and show effects at many developmental stages. 39 Alcohol Figure 5.7 Alcohol-induced craniofacial and brain anomalies in mice Alcohol • There may be several different mechanisms including migration of neural crests cells – prematurely initiate their differentiation into facial bones. • Genes related to the cytoskeleton, which affect migration, are affected by alcohol. • Cells forming median portion of the forebrain, upper midface and cranial nerves may be killed by alcohol. • Cell death may be caused by formation of superoxide free radicals – antioxidants help prevent the effects of alcohol. 41 Alcohol Control Ethanol treated Ethanol + superoxide dismutase Figure 5.8 Cell death caused by ethanol-induced superoxide radicals is a possible mechanism producing fetal alcohol syndrome Neural Crest Cells 43 Alcohol • Both ethanol and acetaldehyde modify the intermediary metabolism of carbohydrates, proteins, and fats. • Both also decrease the transfer of amino acids, glucose, folic acid, zinc, and other nutrients across the placental barrier, indirectly affecting fetal growth due to intrauterine nutrient deprivation. 44 Alcohol 45 Alcohol 46 Alcohol • Alcohol also down-regulates sonic hedgehog (required for formation of brain facial skeleton and organogenesis) • Alcohol can lock neuroblasts into an undifferentiated state by downregulating Sox5 and Ngn1 genes. • Alcohol can induce an increase in DNA methyltransferases activity but not histone transacetylase activity. • Alcohol may affect the cell adhesion protein L1. 47 Figure 5.9 The inhibition of L1-mediated cell adhesion by ethanol is another possible factor in fetal alcohol syndrome Alcohol and the developing brain • The brain undergoes significant structural and functional changes between childhood and adolescence, with maturation continuing in early adulthood. • Two important neurotransmitter systems that undergo substantial changes during adolescence and are affected by alcohol consumption are dopamine and gamma-aminobutyric acid (GABA). Functions of Dopamine •movement •memory •pleasurable reward •behavior and cognition •attention •inhibition of prolactin production •sleep •mood •learning Alcohol and the developing brain • GABA is the primary inhibitory neurotransmitter in the brain— it represses the activity of other brain cells. Alcohol generally enhances the effects of GABA on its receptors, leading to feelings of relaxation and sleepiness. • Gamma-aminobutyric acid (GABA) is the primary inhibitory neurotransmitter known to counterbalance the action of the excitatory neurotransmitter glutamate. • A system using the neurotransmitter glutamate also appears to undergo changes during adolescence. Glutamate interacts with several receptors, including one called the NMDA receptor. Alcohol and the developing brain Difficulties learning new information • One part of the brain that is affected by alcohol is the hippocampus. The hippocampus is a sea horse shaped area deep inside your brain that is responsible for learning and memory. • Alcohol can damage or even destroy the cells that make up the hippocampus, which is why some people experience fuzzy memories or ‘blackouts’ after drinking. Studies have shown that adolescents who drink heavily and often can actually have a smaller hippocampus than their peers. • Damage done to your hippocampus during adolescence can affect your brain’s potential to learn and remember new things for the rest of your life. Teratogenic agents Retinoic acid • It is an important compound involved in development but can disrupt it if it is in the wrong amount or at the wrong times. • RA is important for formation of the anterior-posterior axis in mammals and for heart and jaw formation. • If present in high amounts, cells usually not exposed to it will respond to it. 52 Retinoic acid • Isotretinoin (13-cis-retinoic acid) Accutane was used for treatment of acne in the early 80’s. • Of 59 pregnant women exposed to Accutane • 26 had no noticeable anomalies • 12 aborted spontaneously • 21 were born with anomalies • The main known teratogenic effects of retinoids are face, skull, cardiovascular, nervous system and thymic abnormalities. 53 RA treated Control Figure 5.10 Effects of retinoic acid (RA) on mouse embryos Retinoic acid • RA can disrupt development through different mechanisms. • Alters expression of HOX genes (genes involved in anteriorposterior axis and neural crests cells). • Binds to neural crests cells and inhibits migration. • Activates RA degrading enzymes causing a deficiency – which causes similar anomalies. Teratogenic agents Retinoic acid and public health • RA is a significant public concern because women of childbearing age are the ones using acne meds. • Excess vit A supplementation > 1000 IU/day had a 2% increase of malformations. • Women require a pregnancy test before taking Accutane. • The herbicide Glyphosate may upregulate the activity of endogenous RA. • Phenotypes produced by Glyphosate based herbicides are mainly a consequence of the increase of endogenous retinoid activity. This is consistent with the decrease of Sonic hedgehog (Shh) signaling from the embryonic dorsal midline, with the inhibition of otx2 expression and with the disruption of cephalic neural crest development. 56 Other teratogenic agents Tobacco smoking • Retards the growth of human fetuses and increases risk of fetal and newborn death. • Nicotine may damage fetal brain and lungs during development. • Nicotine induces abnormalities in synapse formation and cell survival. • Carbon monoxide in tobacco smoke can keep the developing baby from getting enough oxygen. • Tobacco smoke also contains other chemicals that can harm unborn babies. Am J Respir Crit Care Med. 2016 Mar 1; 193(5): 486–494. The Role of Nicotine in the Effects of Maternal Smoking during Pregnancy on Lung Development and Childhood Respiratory Disease. Implications for Dangers of E-Cigarettes. Eliot R. Spindel1 and Cindy T. McEvoy2 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4824926/ 57 Other teratogenic agents • • • • • Nicotine may alter the metabolism of fetal lung cells. Increases the risk of lung problems later in life. Lowers sperm in smoking men (>4 cigarettes/day). May affect methylation pattern of tumor suppressor genes. Teenagers of moms who smoked during pregnancy had increased methylation of BDNF genes. • By binding to nicotinic acetylcholine receptors in the brain, nicotine elicits its psychoactive effects and increases the levels of several neurotransmitters in various brain structures – acting as a sort of "volume control". 58 Other teratogenic agents Marijuana • Cannabinols may impair testes function in rats by lowering gonadotropin-stimulating hormone synthesis. • No real evidence of teratogenicity but may have effects on the brain which are only observed later in life. • Cannabinoids altered migration of neuroblasts in rat cerebral cortex which may explain cognitive and memory decline in the adult rats. • Marijuana may be a subtle teratogen that alters brain development. 59 Endocannabinoid system • The cannabinoid receptors are G protein-coupled receptors that are activated by endocannabinoids or exogenous agonists such as tetrahydrocannabinol. • Cannabinoid receptors are found in the parts of the brains involved in emotional and behavioral reactions, homeostasis, learning, memory, and decision-making. • THC may help reduce anxiety however, the use of cannabis in treating anxiety disorders may have adverse effects, such as addiction and cognitive impairment. • It is also consistent with animal experiments showing THC’s ability to "prime" the brain for enhanced responses to other drugs. Alcohol and nicotine also prime the brain for a heightened response to other drugs. 60 Endocannabinoid system • The EC system communicates its messages “backward.” When the postsynaptic neuron is activated, cannabinoids are made “on demand” from lipid precursors (fat cells) already present in the neuron. Then they are released from that cell and travel backward to the presynaptic neuron, where they attach to cannabinoid receptors. • They can control what happens next when these cells are activated. In general, cannabinoids function like a “dimmer switch” for presynaptic neurons, limiting the amount of neurotransmitter (e.g., dopamine) that gets released. • When a person smokes marijuana, THC overwhelms the EC system, quickly attaching to cannabinoid receptors throughout the brain and body. This interferes with the ability of natural cannabinoids to do their job of fine-tuning communication between neurons, which can throw the entire system off balance. 61 Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system, which is involved in a variety of physiological processes including appetite, pain-sensation, mood, and memory. 62 Long-term effects • Rats exposed to THC before birth, soon after birth, or during adolescence show notable problems with specific learning and memory tasks later in life. • Cognitive impairments in adult rats exposed to THC during adolescence are associated with structural and functional changes in the hippocampus. • Frequent use starting in adolescence was associated with a loss of an average of 6 or up to 8 IQ points measured in mid-adulthood. Those who used marijuana showed a significant decline in general knowledge and in verbal ability. • Over time THC can change how the EC system works, which can lead to problems with memory, addiction, and mental health. 63 Prescription drugs Category A Adequate and well controlled studies have failed to demonstrate a risk to the fetus in the first trimester of pregnancy (and there is no evidence of risk in later trimesters). Category B Animal reproduction studies have failed to demonstrate a risk to the fetus and there are no adequate and well-controlled studies in pregnant women. Category C Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant the use of the drug in pregnant women despite potential risks. 64 Prescription drugs Category D There is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks. Category X Studies in animals or humans have demonstrated fetal abnormalities and/or there is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience, and the risks involved in use of the drug in pregnant women clearly outweigh potential benefits. 65 Prescription Drugs Mechanisms • A review of the literature by van Gelder et al, 2010 for mechanisms causing major structural defects caused by prescription drugs commonly taken by women of reproductive age found the following mechanisms. Dr. Ron Saulnier HSS 4102 Fall 2020 • • • • • • Folate antagonism Neural crest disruption Endocrine disruption Oxidative stress Vascular disruption Specific receptor or enzyme-mediated 66 Van Gelder, M., van Rooij,I., Miller R., Zielhuis,G., de Jong-van den Berg L., and Roeleveld,N. Teratogenic mechanisms of medical drugs. Hum Reprod Update. 2010. 16(4):378-94. Read this paper for the details and examples of teratogenic mechanisms https://www.nvp-volumes.org/p2_4.htm More information on teratogens 67 Angiotensin-converting enzyme (ACE) inhibitors • Angiotensin-converting enzyme (ACE) inhibitors can cause fetal growth restriction and fetal death. • Use in late pregnancy is associated with fetal toxicity and intrauterine renal insufficiency and hyperkalemia • Limb contractures, lung hypoplasia, facial anomalies, prematurity are also reported. • Effects are related to the hemodynamic effects on the fetus. • Teratogenicity during the first trimester is low. 68 Warfarin • Warfarin, a blood-thinning drug, inhibits the vitamin K-dependent synthesis of biologically active forms of the calcium-dependent clotting factors II, VII, IX and X • It can cause central nervous system defects, including mental retardation, as well as problems with the optic nerves and hearing. • Nasal hypoplasia and calcific stippling of the epiphyses • Critical period is between 6-9 weeks • Associated with a high risk of miscarriages • Mechanism is of CNS damage is hemorrhage 69 Valproic acid • VA inhibits activity of histone deacetylases – causes hyperacetylation. Many of these genes are regulated by RA. • VA may cause autism-like developmental problems as a result of the effect on histone deacetylases. • VA causes both structural and functional birth defects • VA blocks folate from being absorbed by the embryo leading to neural tube defects. • VA decreases the level of PAX1 transcription in chick somites causing defects in ribs and vertebrae. 70 Other teratogens Pathogens • Viruses are also teratogenic. • Rubella virus makes a protein which stops mitosis by blocking kinases required needed for cell division. • First 5 weeks are the most critical. 71 Pathogens C: Chickenpox and shingles H: Hepatitis B, C, D, E E: Enteroviruses, a group of viruses including poliovirus A: AIDS P: Parvovirus B19, also known as fifth disease T: Toxoplasmosis O: Other infections such as group B streptococcus, listeria, candida R: Rubella C: Cytomegalovirus H: Herpes simplex virus E: Everything else sexually transmitted such as gonorrhea and chlamydia S: Syphilis 72 Pathogens • CMV and Herpes are almost always fatal in early embryos. • In late embryos – cause blindness, deafness, cerebral palsy and mental deficiencies. Heat • An extended maternal temperature of 102F (38.9 C) or higher during the first 6 weeks may affect closure of the neural tube. 73 Herpes Simplex • HSV can be a serious and potentially fatal condition for the fetus. While neonatal herpes is a serious condition, it is also very rare. • Less than 0.1% of babies born in the US each year get neonatal herpes. By contrast, some 25-30% of pregnant women have genital herpes. • Babies are most at risk for neonatal herpes if the mother contracts genital herpes late in pregnancy because a newly infected mother does not have antibodies against the virus, and a new herpes infection is frequently active, so there is an increased risk of the virus being present in the birth canal during delivery. • Women who acquire genital herpes before they become pregnant have a very low risk of transmitting the virus to their babies. • Herpes can also be spread to the baby in the first weeks of life if he or she is kissed by someone with an active cold sore (oral herpes). http://www.ashasexualhealth.org/stdsstis/herpes/herpes-and-pregnancy/ Toxoplasmosis • Toxoplasmosis normally causes a mild illness in people with healthy immune systems, it's risky during pregnancy because it may harm your baby. • The parasite can be found in meat, cat faeces, the soil where cats defecate and unpasteurised goats’ milk. • Toxoplasmosis is only a risk to an unborn baby if caught for the first time during pregnancy or within a few weeks before you get pregnant. The damage the infection may cause will depend on when in pregnancy you got the infection. • On average, only 4 in 10 of such infections will pass to the baby. Caught during pregnancy, toxoplasmosis can cause miscarriage, stillbirth or damage to the baby’s brain and the eyes. • However, most babies born with toxoplasmosis have no obvious damage at birth but develop symptoms, usually eye damage, during childhood or even adulthood. A few will have more serious symptoms such as blindness or brain damage. Cytomegalovirus • Cytomegalovirus (CMV) is a common virus in the herpes virus family. 50% of people have been infected by young adulthood and up to 85% by age 40. • It is rare for a person to get symptoms after the initial infection unless their immune system is weakened by severe illness and treatments (e.g. for cancer). • Reactivation can occur during pregnancy in women who have had infection previously, with a very small risk of transmission of CMV to the unborn baby. • If a woman is newly infected with CMV while pregnant, there is a risk that her unborn baby will also become infected (congenital CMV). • Hearing loss is the most common sign of congenital CMV. However, some infants with congenital CMV infection who appear healthy at birth develop hearing or vision loss over time. https://www.health.nsw.gov.au/Infectious/factsheets/Pages/cmv-and-pregnancy.aspx