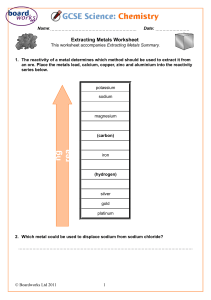
SPN 21 CHEMISTRY SCHEME OF WORKS 2 Years Program 1 SPN 21 CHEMISTRY 5070 SCHEME OF WORKS YEAR 9 TOPIC TITLE NO. OF WEEKS 1 Introduction to Chemistry 1 2 Kinetic Particle Theory 2 3 Atomic Structure 2 4 Chemical Bonding 3 5 Chemical Formulae 2 6 Types of Common Chemical Reactions 4 7 Stoichiometry and Mole Concept 4 8 Experimental Chemistry 3 9 Acids, Bases and Neutralisation 4 10 Salts 3 11 Qualitative Analysis 4 12 Metals and Extraction 4 Total 2 36 TOPIC 1: INTRODUCTION TO CHEMISTRY Duration: 1 weeks Learning outcomes: Students should be able to: define chemistry. explain that chemists investigate (i.e. learn about) substances. describe the scientific method used in chemistry. reason out why study chemistry. Topic / Sub-topic TOPIC 1 Introduction to Chemistry Importance of chemistry Introduction of the first 20 elements No. of Weeks Lesson Objectives Students should be able to: Suggested Activities Resources Activity 1.1 Short briefing on chemistry related career. Activity 1.2 Safety in the lab and hazard symbols. Activity 1.3 Chemistry in our life. (a) Understand chemistry and its importance. (b) Name and recognise the symbols of the first 20 elements in the Periodic Table. 1 Activity 1.4 Use mnemonics to familiarize with names and symbols of first row of the common transition metals. 3 http://www.chymist .com/Measuremen t.pdf http://ucdsb.on.ca/t iss/stretton/CHEM 1/ametricx.html http://www.physics .nist.gov/Genint/Ti me/time.html TOPIC 2: KINETIC PARTICLE THEORY Duration: 2 weeks Prior Knowledge: States of Matter (Solid, liquid and gas) Links to: LSS – Matter, Topic 17 – Speed of Reactions Keywords: boiling, condensation, evaporation, freezing, melting, sublimation, boiling point, melting point, freezing point, diffusion, change of state, kinetic theory, element, mixture, compound. Misconception: 1. 2. 3. 4. 5. Diagrammatic representation of liquid must show particles to be loosely arranged but in contact with one another. Gas must be randomly arranged, must show no pattern. Liquid cannot be compressed as there are small spaces between the particles. Particles in solid are not moving. Movement does not mean moving from one place to another. Learning outcomes: Students should be able to: draw the arrangement of particles in solid, liquid and gas. give the explanation of melting, freezing, evaporation, condensation, boiling and sublimation. state that particles in a solid vibrate at their fixed positions. state that particles in a liquid can move freely within the container. state that particles in gas move freely at a high speed . give the reason why solid and liquid cannot be compressed, liquid can flow and gas can exert pressure. state why as the temperature is increased, the movement of the particles becomes faster and the pressure becomes greater. 4 state the evidences for the movement of particles in liquids and gases. define diffusion and state the effects of diffusion in terms of kinetic particle theory. give examples of diffusion in everyday life. state qualitatively the effect of molecular mass on the rate of diffusion and the effect of temperature on the rate of diffusion. Define elements, mixtures and compounds and give their diagrammatic representation. Topic / Sub-topic TOPIC 2 Kinetic Particle Theory States of matter A theory of matter Particulate models of matter Changes in states No. of Weeks Lesson Objectives (a) Describe the solid, liquid and gaseous states of matter and explain their interconversion in terms of the kinetic particle theory and of the energy changes involved. Activity 2.2 Using role play to demonstrate the movement of particles in solid, liquid and gas. (b) Describe and explain the evidence for the movement of particles in liquids and gases. (d) State qualitatively the effect of molecular mass on the rate of diffusion and explain the dependence of rate of diffusion on temperature. Heating / cooling curves (e) Describe the heating / cooling curves of a substance. Elements, mixtures, and compounds (f) Describe the differences compounds and mixtures. between elements, 5 Resources Activity 2.1 Demonstration: Using kinetic particles theory model. Students should be able to: (c) Explain everyday effects of diffusion in terms of particles, e.g. the spread of perfumes and cooking aromas; tea and coffee grains in water. Suggested Activities 2 Activity 2.3 Experiment: To determine the melting point of naphthalene using cooling curve. Activity 2.4 Demonstration: To determine the purity of ethanol by determining its boiling point. Activity 2.5 Diagrammatic representation mixtures, and compounds. of elements, http://youth.net/nsr c/sci/sci023.html#a nchor1265203 http://www.uky.edu /Projects/Chemco mics/ http://www.science .co.il/PTelements.a sp?s=Discovery http://www.levity.c om/alchemy/egypti on_symbols.html http://www.levity.c om/alchemy/val_sy mb,html http://www.levity.c om/alchemy/daltin _s.html TOPIC 3: ATOMIC STRUCTURE Duration: 2 weeks Links to: Physics – Atomic Physics, Topic 4 – Chemical bonding Keywords: anion, atom, atomic number, atomic structure, cation, electron, electron shell, electronic structure, electronic configuration, ion, isotopes, mass number, neutral, neutron, nucleon number, nucleus, period, Periodic Table, proton, proton number, symbol, valence electron, valency. Learning outcomes: Students should be able to: draw the atomic structure of an atom showing the shells, the electrons orbiting the nucleus and the protons and neutrons inside the nucleus. define proton, neutron and electron. state the relative charges and approximate relative masses of a proton, a neutron and an electron. draw the atomic structures of the first 20 elements in the Periodic Table. define proton number and nucleon number. use the Periodic Table to obtain the proton number and nucleon number of an element. calculate the number of neutron of an atom or an ion using the formula; Nucleon number = number of proton + number of neutron. -6- define isotopes. state radioactive isotopes, give some common examples and their uses. state the stable electron configuration (electron configuration of Group O). describe the formation of positive ions by loss of electrons in metal atoms (Li, Be, Na, Mg, Al, K and Ca) to achieve stable electron configuration. describe the formation of negative ions by gain of electron in non-metal atoms (F, Cl and O) to achieve stable electron configuration. work out the number of sub-atomic particles present in positive ions (cations) and negative ions (anions). Topic / Sub-topic TOPIC 3 Atomic Structure Introduction to Periodic Table Protons, neutrons and electrons The structure of an atom. No. of Weeks Lesson Objectives Activity 3.1 Demonstration: Using optic chart viewer to show the structure of an atoms (if available). Students should be able to: (a) State the relative charges and approximate relative masses of a proton, a neutron, and an electron. (b) Describe with the aid of diagrams, the structure of an atom as containing protons and neutrons (nucleons) in the nucleus and electrons arranged in shell (energy levels) (no knowledge of s, p, d, f classification will be expected). 2 (e) Deduce the number of protons, neutrons, and electrons in atoms and ions from protons and nucleon numbers. (f) Define the term isotopes. (g) State that some isotopes are radioactive. Ions (d) Interpret and use the symbols such as 126 C . Isotopes Resources (c) Define proton number and nucleon number. Suggested Activities (h) Describe the formation of ions by electron loss/gain in order to obtain the electronic configuration of an inert gas. 7 http://molaire1.club .fr/e_histoire.html http://www.aip.org/ history/electron/jjho me.htm http://web.visionlea rning.com/custom/ chemistry/animatio ns/CHE1.2-anatoms.shtml http://www.chem4k ids.com/files/atom_ isotopes.html http://www.chem4k ids.com/files/atom_ structure.html http://www.chem4k ids.com/files/eleme nts/index.html TOPIC 4: CHEMICAL BONDING Duration: 3 weeks Prior Knowledge: Topic 3 – Atomic Structure Links to: Topic 5 – Chemical Formulae Keywords: electron transfer, covalent bond, covalent compound, dot and cross diagrams, double bond, ionic bond, ionic compound, binary compound. Learning outcomes: Students should be able to: define ionic bonding, ionic bonds and ionic compounds. state the formation of ions by electron loss/gain in order to obtain the electron configuration of a noble gas. state that ionic bonds are formed between metals and non-metals. draw dot and cross diagram to show the bonding in ionic compounds. state the bonding in sodium chloride which contains a giant lattice in which the ions are held by electrostatic attraction. deduce the formulae of other binary ionic compounds from diagrams of their lattice structures. state the physical properties of ionic compounds and relate the properties to their lattice structures. define covalent bonding, covalent bonds, covalently bonded elements and covalent compounds. state the formation of covalent bond by the sharing of a pair of electrons in order to gain the electron configuration of a noble gas. draw dot and cross diagrams to show the covalent bonding in molecules. 8 state that covalent bonds are formed between non-metallic elements such as in H 2 ; Cl 2 ; O 2 ; HCl ; N 2 ; H 2 O ; CH 4 ; C 2H 4 ; CO 2 and other molecules. state the physical properties of covalent molecules and relate the properties to their structures and bonding. define molecular substances and giant molecular substances. give examples of molecular substances and giant molecular substances. state the structures and bonding of molecular substances and giant molecular substances and relate to their physical and chemical properties. draw the structure of metals by showing the lattice of positive ions in a “sea of electrons”. state the physical properties of metals. relate the physical properties of metallic elements such as malleability to their structures and the electrical conductivity to the mobility of the electrons in the structure. Topic / Sub-topic TOPIC 4 Chemical Bonding Ionic bonding No. of Weeks Lesson Objectives Suggested Activities Activity 4.1 Demonstration: To show the ionic bonding by burning magnesium in air (oxygen). Students should be able to: (a) Describe the formation of ionic bonds between metals and non-metals, e.g. NaCl ; MgCl 2 . Activity 4.2 Practice on drawing diagrams of ionic and covalent compounds. (b) State that ionic materials contain a giants lattice in which the ions are held by electrostatic attraction, e.g. NaCl (students will not be required to draw diagram of ionic lattice). Resources (c) Deduce the formula of the other ionic compounds from diagrams of their lattice structures, limited to binary compounds. (d) Relate the physical properties (including electrical property) of ionic compound to their lattice structure. Covalent bonding (e) Describe the formation of a covalent bond by the sharing of a pair of electrons in order to gain the electronic configuration of an inert gas. Describing, using ‘dot and cross’ diagrams, the formation of covalent bonds between non-metallic H 2 ; Cl 2 ; O 2 ; HCl ; N 2 ; elements, e.g. H 2 O ; CH 4 ; C 2 H 4 ; CO 2 (g) Deduce the arrangement of electrons in other covalent molecules. 3 (f) (h) Relate the physical properties (including electrical properties) of covalent compounds to their structure and bonding. 9 http://web.jjay.cuny .edu/~acrpi/NSC/5bonds.htm http://www.dac.neu .edu/physics/b.mah eswaran/phy1121/ data/ch09/anim/ani m0904.htm http://www.bbc.co. uk/schools/gcsebit size/chemistry/clas sifyingmaterials/ion ic_bondingrev5.sht ml http://www.bbc.co. uk/schools/gcsebit size/chemistry/clas sifyingmaterials/co valent_bondingrev 3.shtml http://ithacascience zone.com/chemzo ne/lessons/03bondi ng/mleebonding/m etallicbonding.htm http://www.acdlabs .com/products/che m_dsn_lab/chemsk etch/ http://www.sucessli nk.org/colearn/cl_l esson.asp?offset=1&lid=4378 Topic / Sub-topic Metallic bonding Structure and properties of materials No. of Weeks Lesson Objectives Suggested Activities Resources (i) Describe metals as a lattice of positive ions in a ‘sea of electrons’ Activity 4.3 Build crystal lattice of NaCl and MgCl 2 (j) Relate the malleability of metals to their structure and the electrical conductivity of metals to the mobility of the electrons in the structure. Activity 4.4 Show models of diamond and graphite. (k) Compare the structure of molecular substances, e.g. methane, iodine, with those of giant molecular substances, e.g. poly(ethene); sand; diamond; graphite in order to deduce their properties. (l) Compare the bonding and structure of diamond and graphite in order to deduce properties such as electrical conductivity, lubricating or cutting action (students will not be required to draw the structure). (m) Deduce the physical and chemical properties of substances from their structures and bonding and vice versa. 10 http://www.rdg.ac.u k/~scsharip/tube.ht m http://www.pa.msu. edu/cmp/csc/nanot ube.html TOPIC 5: CHEMICAL FORMULAE Duration: 2 weeks Prior Knowledge: Topic 3 – Atomic Structure, Topic 4 – Chemical Bonding Links to: Topic 6 – Types of Common Chemical Reactions, Topic 7 – Stoichiometry and Mole Concept, Topic 9 – Acids, Bases and Neutralisation, Topic 10 – Salts Keywords: binary compounds, covalent compound, diatomic molecule, valency, monovalent ion, divalent ion, trivalent ion. Learning outcomes: Students should be able to: state the formulae of common positive ions. state the formulae of common negative ions. state that the ionic compounds are made up of positive and negative ions. use valency to write the formula of a compound. state that metallic element precedes the non-metallic element in writing the formula of ionic compound. state that the total sum of charges in an ionic compound must equal to zero. apply cross method using valency to derive the formulae of ionic compounds. 11 write the number of atoms as subscript on the right. ignore subscript ‘1’ if the number of atom is 1. use bracket for polyatomic ions, e.g. CaOH2 . count the number of atoms of each element in a compound. state the valency of elements from the structural formula of covalent compound. Topic / Sub-topic TOPIC 5 Chemical Formulae Formula of ionic and covalent compounds No. of Weeks Lesson Objectives Activity 5.1 To work out the formula of ionic compound using card games. Students should be able to: (a) State the symbols of the elements and formulae of the compounds mentioned in the syllabus. (b) Deduce the formula of simple compound from the relative numbers of atoms present and vice versa. (c) Deduce the formula of simple ionic compounds from the charges on the ions present and vice versa. 12 Suggested Activities 2 Resources TOPIC 6: TYPES OF COMMON CHEMICAL REACTIONS Duration: 4 weeks Prior Knowledge: Topic 5 – Chemical Formula Links to: Topic 8 – Experimental Chemistry, Topic 9 – Acids, Bases and Neutralisation, Topic 10 – Salts, Topic 12 – Metals and Extraction, Topic 13 – The Periodic Table Keywords: reactivity series of metals, direct combination reaction, solubility of salt, neutralization, metal, acid, carbonate, precipitation reaction, displacement reaction, thermal decomposition, direct reaction, chemical equation, ionic equation, word equation. Learning outcomes: Students should be able to: state whether a salt is soluble or insoluble by referring to the general rules of solubility. write word equation for a given reaction. state the products formed from various types of chemical reactions relate that thermal decomposition of carbonate leads to the production of gas and an oxide. test gas produced and observe colour change of solid in the thermal decomposition of carbonate. observe the difference in physical and chemical properties between binary compound from its constituents. 13 write formulae of simple covalent and ionic compounds including formulae of non-metallic elements. balance a chemical equation for a given reaction. recognize that only soluble ionic substances will be able to dissociate for ionic equations. eliminate spectator ions in the chemical equation to obtain the ionic equation. balance total charges of reactants and products in an ionic equation. Topic / Sub-topic TOPIC 6 Types of Common Chemical Reactions Introduction to Reactivity Series of Metals. Solubility of salt Neutralization Metal + water Metal + acid Acid + carbonate reaction No. of Weeks Lesson Objectives Suggested Activities Activity 6.1 Practical: To show neutralisation between dilute hydrochloric acid and dilute sodium hydroxide (using various indicators including phenolphthalein). Students should be able to: (a) Describe the general rules of solubility of common salts include nitrates, chlorides (including silver and lead), sulphates (including barium, calcium and lead), carbonates, hydroxides, Group I cations and ammonium salts. (b) Describe and give examples of different types of common chemical reactions. 4 Activity 6.2 Practical: To show relative reactivity of metals with water. Safety: A very small amount of potassium and sodium to be used in this reaction. Safety screen or goggles are advised. Practical Skill: Be able to describe and observe accurately and compare the degree of reactivity among the different metals. Activity 6.3 Practical: To show reaction between metals and dilute hydrochloric acid. Safety: Do not use potassium or sodium. Activity 6.4 Practical: To show reaction of carbonates with dilute hydrochloric acid. 14 Resources Topic / Sub-topic Precipitation reaction Displacement reaction Thermal decomposition No. of Weeks Lesson Objectives Suggested Activities Activity 6.5 Practical: To show precipitation reactions. Practical Skill: Observe precipitation from adding two solutions. Activity 6.6 Practical: To show displacement reactions between metals. Practical Skill: observe colour change of solution and deposit formed on the surface of the original metal. Activity 6.7 Practical: To show displacement reaction between halogens. Activity 6.8 Practical: To show thermal decomposition of carbonates. Direct reaction Activity 6.9 Practical: To show direct reaction by heating. Chemical equation Ionic equation (d) Interpret and construct chemical equations, with state symbols, including ionic equation. 15 Resources TOPIC 7: STOICHIOMETRY AND MOLE CONCEPT Duration: 4 weeks Prior Knowledge: Identify Atomic Mass from Periodic Table, deduce Chemical Formula (ionic and covalent), balancing chemical equation. Links to: Topic 6 – Types of Common Chemical Reactions Keywords: relative molecular mass, (Mr), empirical formula, molecular formula, moles, mole ratio, molar mass, molar volume, molar concentration, Avogadro’s number (though not in syllabus, important concept), limiting reagent, excess reagent, % yield, % purity. Misconception: In stoichiometry the ratio for reacting substances is moles to moles instead of mass to mass, molar volume of gas (24 dm 3 per mole at r.t.p) is often used even for solution. Learning outcomes: Students should be able to: calculate empirical formula, given % by mass or mass itself, work out the molecular formula given the molecular mass and empirical formula which was deduced. calculate the number of moles given either mass, volume of gas, or concentration and volume of solution. use mole ratio to answer the question asked. deduce the limiting reagent and hence the yield expected given the amount of both reactants. calculate number of moles given the concentration and volume of solution. 16 relate titration results to calculations. Convert concentration mol dm 3 to g dm 3 and vice versa. deduce mass of theoretical yield in question and mass of impurity in question. M V apply a a x , where x is the mole ratio of the reacting solutions. M b Vb Topic / Sub-topic TOPIC 7 Stoichiometry and Mole Concepts Relative atomic mass Relative molecular (or formula) mass Avogadro’s number ** (** although not in syllabus but it is an important chemistry concept) No. of Weeks Lesson Objectives Students should be able to: (a) Deduce the formula of simple compound from the relative numbers of atoms present and vice versa. (b) Define relative atomic mass, Ar . (c) Define relative molecular mass, M r , and calculate relative molecular mass (relative formula mass) as the sum of relative atomic masses. (d) Calculate the percentage mass of an element in a compound when given appropriate information. Molar mass Molar volume Molar concentration Empirical formula Limiting reactants (e) Calculate empirical and molecular formulae from relevant data. (f) Calculate stoichiometric reacting masses and volumes of gases (one mole of gas occupies 24 dm 3 at room temperature and pressure); calculating involving the idea of limiting reactants may be set (questions on the gas laws and the calculation of gaseous volumes at different temperatures and pressures will not be set). (g) Apply the concept of solution concentration (in Percentage yield and percentage purity mol/dm 3 or g/dm 3 ) to process the results of volumetric experiments and to solve simple problems (appropriate guidance will be provided where unfamiliar reactions are involved) (h) Calculate % yield and % purity. 17 4 Suggested Activities Resources Activity 7.1 Practical: To prepare standard solution of copper(II) sulphate. Activity 7.2 Experiment: To determine the percentage purity of sodium carbonate in a mixture of sodium carbonate and ammonium carbonate. http://www.carlton. paschools.pa.sk.ca /chemical/Molemas s/default.htm http://www.carlton. paschools.pa.sk.ca /chemical/Molemas s/moles6.htm TOPIC 8: EXPERIMENTAL CHEMISTRY Duration: 3 weeks Prior Knowledge: Topic 6 – Types of Common Chemical Reactions Links to: Topic 9 – Acids, Bases and Neutralisation, Topic 10 - Salt Keywords: solute, solvent, solution, filtration, filtrate, residue, crystallisation, simple distillation, fractional distillation, chromatography, chromatogram, decantation. Misconception: 1. The common misconception is that all salts are soluble in water. This could be due to mistaking the word salt to mean table salt, which is soluble. 2. Air is not necessarily in the gaseous form all the time. It can be liquefied and fractionally distilled. Safety: Take care while separating ethanol by fractional distillation. It catches fire easily. Learning outcomes: Students should be able to: state the method of separating soluble and insoluble substances by filtration. state the method of separating solvent from solution by simple distillation. state the method of separating miscible liquids by fractional distillation. state the method of separating immiscible liquids by using separating funnel. 18 suggest a suitable method of separation given the information about the substances involved. describe the separation of petroleum fractions by fractional distillation. describe the method of separating substances by chromatography and calculate the R f value. Topic / Sub-topic TOPIC 8 Experimental Chemistry Separation techniques No. of Weeks Lesson Objectives Activity 8.1 Practical: To obtain copper(II) sulphate crystals from a mixture of copper(II) sulphate and sand. Students should be able to: (a) Name appropriate apparatus for the measurement of time, temperature, mass and volume, including burettes, pipettes, measuring cylinders and gas syringes. Activity 8.2 Demonstration on decanting and using separating funnel. (b) Suggest suitable apparatus, given relevant information, for a variety of simple experiments, including collection of gases and measurement of rates of reaction. Tests of purity Activity 8.3 Demonstration on simple distillation (using salt solution). (c) Describe methods of purification by the use of a suitable solvent, filtration and crystallisation, distillation and fractional distillation, with particular references to the fractional distillation of crude oil, liquid air and fermented liquor. (d) Suggest suitable methods of purification, given information about the substances involved. (e) Describe paper chromatography and interpret chromatograms including comparison with ‘known’ samples and the use of R f values. (f) Explain the need to use locating agents in the chromatography of colourless compounds. (g) Deduce from the given melting point and boiling point the identities of substances and their purity. (h) Explain that the measurement of purity in substances used in everyday life, e.g. foodstuffs and drugs, is important. 19 Suggested Activities Activity 8.4 Demonstration on fractional distillation (using ethanol and water). 3 Activity 8.5 Experiment: To separate various dyes in food colouring and measure the R f values. Resources TOPIC 9: ACIDS, BASES AND NEUTRALIZATION Duration: 4 weeks Links to: LSS – Acid and Alkali, Topic 5 – Chemical Formulae, Topic 6 – Types of Common Chemical Reaction Keywords: strong acid, weak acid, complete dissociation, partial dissociation, hydrogen ions, hydroxide ions, neutralization, acidity, alkalinity, neutral oxide, acidic oxide, basic oxides, amphoteric oxides, acidic soil, lime Misconception: Not necessary the reaction between an acid and an alkali will end up neutral. The amounts of the reacting substances need to be considered. Safety: 1 2 3 4 Wash hand thoroughly when in contact with alkalis or acids. Do not fill the pipette by sucking with the mouth, use pipette filler. Be careful not to suck the solution into the pipette filler, this will spoil the filler. Use goggles during heating. Learning outcomes: Students should be able to: define acid. state the formula of common ion present in all acid. give some examples of acids. state the physical properties of acid; its taste, pH values, effects on litmus paper and universal indicator paper. define base and alkali and give examples. describe the reaction of acids and metals. describe the reaction of acids and bases. describe the reaction of acids and carbonates. state the difference between a strong and a weak acid. 20 construct and write ionic equation for neutralisation reaction. explain why soil becomes acidic. describe how to treat acidic soil. give some uses of acid. state the physical properties of alkali; its taste, pH values, effect on litmus paper and universal indicator paper. describe the reaction of alkali with ammonium salts. give some uses of alkali. classify oxides as acidic, basic, amphoteric and neutral. Topic / Sub-topic TOPIC 9 Acids, Bases, and Neutralization The characteristics properties of acids and bases Acid – base titration No. of Weeks Lesson Objectives Activity 9.1 Practical: To neutralise hydrochloric acid by titrating with sodium hydroxide solution. Students should be able to: (a) Describe the meaning of the terms acid and alkali in term of the ions they contain or produce in aqueous solution and their effect on universal indicator paper. Activity 9.2 Practical: To titrate sodium carbonate and hydrochloric acid and to find percentage purity of sodium carbonate (b) Describe how to test hydrogen ion concentration and hence relative acidity using universal indicator paper and the pH scale. Activity 9.3 Experiment: To show reaction between sodium hydroxide and ammonium chloride. (c) Describe the characteristics properties of acids as in reactions with metals, bases and carbonates. (d) Describe qualitatively the difference between strong and weak acids in term of the extent ionisation. (e) Describe neutralisation as a reaction between hydrogen ions and hydroxide ions to produce water. H OH - H 2 O (f) Describe the importance of controlling the pH in soils and how excess acidity can be treated using calcium hydroxide. (g) Describe the characteristics properties of bases in reaction with acid and with ammonium salts. Types of oxides (h) Classify oxides as acidic, basic and amphoteric, based on metallic/non-metallic character. 21 Suggested Activities 4 Resources http://www.levity.co m/alchemy/symaci ds.html/ TOPIC 10: SALTS Duration: 3 weeks Links to: Topic 8 – Experimental Chemistry, Topic 5 – Chemical Formulae, Topic 6 – Types of Chemical, Topic 9 – Acids, Bases and Neutralisation Keywords: soluble salt, insoluble salts, crystals, saturated solution, precipitates, solubility, dissolving, filtration, evaporation, crystallisation, filtrate, residue Misconception: The common misconception is that all salts are soluble in water. This could be due to mistaking the word salt to mean table salt, which is soluble. Learning outcomes: Students should be able to: describe how to prepare copper(II) sulphate crystals by reacting an acid with insoluble base / carbonate. describe how to prepare insoluble salt of silver chloride by precipitation. describe how to prepare soluble salt of sodium chloride by reaction of alkali and acid (titration) use the table of solubility of salts. write a balanced chemical equation for preparation of a named salt. 22 Topic / Sub-topic TOPIC 10 Salts Preparation and purification of salts Precipitation No. of Weeks Lesson Objectives Activity 10.1 Practical: To prepare copper(II) sulphate crystal by reacting sulphuric acid with copper(II) oxide or copper(II) carbonate. Students should be able to: (a) Describe the technique used in the preparation, separation and purification of salts (method of preparation should include precipitation and titration together with reactions of acid with metals, insoluble bases and insoluble carbonates). (b) Suggest a method of preparing a given salt from suitable starting materials, given appropriate information. Suggested Activities Activity 10.2 Practical: To prepare insoluble salt. 3 Activity 10.3 Experiment: water. To investigate solubility of salts in Activity 10.4 Experiment: To determine the solubility of salts in g cm 3 . 23 Resources TOPIC 11: QUALITATIVE ANALYSIS Duration: 4 weeks Prior Knowledge: Topic 5 – Chemical Formula, Topic 10 – Salts Links to: Topic 6 – Types of Common Chemical Reactions Keywords: cations, anions, gases, precipitate, soluble, insoluble, in excess, coloured / colourless solution, effervescence, no visible change, gelatinous, powdery Misconception: Clear is always misconceived as colourless. In fact any coloured solution is clear as long as it allows light to pass through. Learning outcomes: Students should be able to: read and follow the procedures and instructions closely. carry out tests to identify the presence of cations using aqueous sodium hydroxide and aqueous ammonia. describe what is observed when aqueous sodium hydroxide and aqueous ammonia are added to the cations. carry out test to identify the presence of anions (carbonate, chloride, iodide, sulphate and nitrate). name each precipitate formed by the reaction of anions with the respective reagents. describe what is observed during the test, together with the equations for the reactions. describe the test for the common gases and water vapour. 24 Topic / Sub-topic TOPIC 11 Qualitative Analysis Identification of ions No. of Weeks Lesson Objectives Students should be able to: Activity 11.1 Practical: To identify the following cations: (a) Describe the use of aqueous sodium hydroxide and aqueous ammonia to identify the following aqueous cations: aluminium, ammonium, calcium, copper(II), iron(II), iron(III) and zinc (formula of complex ions are not required). Al 3 , NH 4 , Ca 2 , Cu 2 , Fe 2 , Fe 3 , and Zn 2 Activity 11.2 Practical: To identify the following anions: CO 32 , Cl , I , NO 3 , and SO 42 (b) Describe test to identify the following anions: carbonates (by addition of dilute acid and subsequent use of limewater); chloride (by reaction of aqueous solution with nitric acid and aqueous silver nitrate); iodide (by reaction of aqueous solution with nitric acid and aqueous lead(II) nitrate); nitrate (by reduction with aluminium and aqueous sodium hydroxide to ammonia and subsequent use of litmus paper) and sulphate (by reaction of an aqueous solution with nitric acid and aqueous barium nitrate). Identification of gases Suggested Activities (c) Describe test to identify the following gases: ammonia (using damp red litmus paper); carbon dioxide (using limewater); chlorine (using damp litmus paper); hydrogen (using burning splint); oxygen (using a glowing splint) and sulphur dioxide (using acidified potassium dichromate (VI)). (d) Describe a chemical test for water. 25 Activity 11.3 Practical: To test for gases: ammonia, carbon dioxide, chlorine, hydrogen, oxygen and sulphur dioxide. 4 Resources TOPIC 12: METALS AND EXTRACTION Duration: 4 weeks Prior Knowledge: Topic 6 – Types of Common Chemical Reactions Links to: Topic 13 – The Periodic Table Keywords: alloys, reactivity series, thermal stability, displacement reaction, metal ores, sacrificial protection, recycling, galvanizing, corrode preferentially. Learning outcomes: Students should be able to: list out the general physical properties of metals in term of their structure. define alloys. give examples of alloys. draw diagrams to show the representation of pure metals and alloys. state the differences between physical properties of metals and alloys. write the equations for the reactions of metals with water and metals with dilute acids. write equations for the reduction reactions of the metal oxides by carbon or hydrogen. arrange metals in order of their reactivity, most reactive to least reactive. relate reactivity series to the tendency of a metal to form its positive ion. compare the reactivity of metals by displacement reaction. write equations for the action of heat on the carbonates of the metals in the reactivity series. relate thermal stability to the reactivity series. name the methods by which metals are obtained from their ores and relate these to their positions in the reactivity series. define recycling. list out the social, economic and environmental advantages and disadvantages of recycling metals. 26 give examples of common metals that can be recycled. outline the reactions taking place in the blast furnace for the extraction of iron from haematite. state the raw materials needed for the extraction of iron in the blast furnace. sketch the diagram of the blast furnace and label the raw materials input into the furnace and the products collected. state the uses of the pig iron obtained from the extraction and give the uses of the different types of steel made from the iron. relate the uses of the high carbon steel, low carbon steel and mild steel to their physical properties. define rusting. state the conditions needed for corrosion(rusting) to occur. give ways to prevent rusting from taking place (painting, greasing, plastic coating, galvanizing and sacrificial protection) define sacrificial protection. relate how sacrificial protection work to the positions of metals in the reactivity series. state the reason why underwater pipes have a piece of magnesium attached to them. outline the extraction of aluminium (refer to electrolysis). Topic / Sub-topic TOPIC 12 Metals and Extraction Properties of metals Alloys and uses No. of Weeks Lesson Objectives Students should be able to: (a) Describe the general physical properties of metals (as solids having high melting point and boiling points; good conductor of heat and electricity) in term of their structure. (d) Explain why alloys have different physical properties to their constituent elements. Metal + water Metal + acid (ii) Displacement reaction (f) The reduction, if any, of their oxides by carbon and/or by hydrogen. Describe the reactivity series as related to the tendency of a metal to form its positive ion, illustrated by its reaction (i) The aqueous ions of the other listed metals (ii) The oxides of the other listed metals (g) Deduce the other of reactivity from a given set of experimental results. Thermal decomposition (h) Describe the action of heat on the carbonates of the listed metals and relate thermal stability to the reactivity series. 27 Activity 12.1 Experiment: To compare the reactivity of metals by displacement reaction. http://en.wikipedi a.org/wiki/Thermit e http://jchemied.ch em.wisc.edu/JCE Soft/CCA/sample s/cca7thermite.ht ml http://davidavery. co.uk/thermite/ http://www.bbc.co .uk/history/british/ victorians/launch _ani_blast_furnac e.shtml http://www.howst urffworks.com.iro n.htm Activity 12.3 Experiment: carbonates. (c) Identify representation of metals and alloys from diagrams of structures. Resources Activity 12.2 Demonstration: To show Thermit reaction (reduction of metal oxide). (b) Describe alloys as a mixture of a metal with another element, e.g. brass; stainless steel. (e) Place in order of reactivity calcium, copper, (hydrogen), iron, lead, magnesium, potassium, silver, sodium and zinc by reference to (i) The reactions, if any, of the metals with water, steam and dilute hydrochloric acid. Suggested Activities 4 To show action of heat on the Topic / Sub-topic No. of Weeks Lesson Objectives Extraction of metals (i) Describe the ease of obtaining metals from their ores by relating the elements to their positions in the reactivity series. Recycling of metals (j) Describe metal ores as a finite resource and hence the need to recycle metals. (k) Discuss the social, economic and environmental advantages and disadvantages of recycling metals, e.g. aluminium and copper. Iron (l) Describe and explain the essential reactions in the reaction of iron using haematite, limestone and coke in the blast furnace. (m) Describe steels as alloys which are a mixture of iron with carbon or other metals and how controlled use of these additive changes the properties of the iron, e.g. high carbon steels are strong but brittle whereas low carbon steels are softer and more easily shaped. (n) State the uses of mild steel (e.g. car bodies; machinery) and stainless steel (e.g. chemical plant; cutlery; surgical instruments) (o) Describe the essential condition for the corrosion (rusting) of iron as the presence of oxygen and water; prevention of rusting can be achieved by placing a barrier around the metal (e.g. painting; greasing; plastic coating; galvanising) (p) Describe the sacrificial protection of iron by a more reactive metal in terms of the reactivity series where the more reactive metal corrode preferentially (e.g underwater pipes have a piece of magnesium attached to them). Aluminium (refer to electrolysis) 28 Suggested Activities Activity 12.4 Experiment: To determine conditions for rusting. Activity 12.5 Experiment: To show sacrificial protection of metal. Activity 12.6 Experiment: To reduce lead(II) oxide by carbon. Resources SPN 21 CHEMISTRY 5070 SCHEME OF WORKS YEAR 10 TOPIC TITLE NO. OF WEEKS 13 The Periodic Table 2 14 Energy from Chemicals 3 15 Electrolysis 4 16 Speed of Reaction 4 17 Reversible Reactions 3 18 Redox 4 19 Atmosphere and Environment 2 20 Organic Chemistry 6 Total 29 28 TOPIC 13: THE PERIODIC TABLE Duration: 2 weeks Links to: Topic 3 – Atomic Structure Keywords: period, group, group property, periodic trend, Metallic/non-metallic character, alkali metal, transition metal, halogen, monatomic, diatomic, variable valency. Learning outcomes: Students should be able to: describe how the elements are arranged in the Periodic Table. describe how the position of an element in the Periodic Table is related to the proton number and electronic structure. identify the metals and non-metals from the Periodic Table. describe the relationship between group number to the number of valence electrons in an element. describe the relationship between period number to the number of shell in an element. describe the change from metal to non-metal across the Periods from left to right. 30 describe the relationship between group number to the ionic charge for an element (especially for metals in Group I, II and III; non-metals in Group VII, VI). describe the main physical properties of alkali metals, halogens and noble gases. describe the trend in physical properties down the groups for alkali metals and halogens. describe the trend in chemical properties of Group I and Group VII. describe the main properties of the transition metals. describe the unreactivity of the noble gases. state the main uses of the noble gases. Topic / Sub-topic TOPIC 13 The Periodic Table Periodic trends No. of Weeks Lesson Objectives Students should be able to: (a) Describe the Periodic Table as an arrangement of the elements in the order of increasing proton (atomic) number. (b) Describe how the position of an element in the Periodic Table is related to proton number and electronic structure. (c) Describe the relationship between Group number and the ionic charge of an element. (d) Explain the similarities between the elements in the same Group of the Periodic Table in terms of their electronic structure. (e) Describe the change from metallic to non-metallic character from left to right across a period in the Periodic Table. (f) Describe the relationship between Group number, number of valency electrons and metallic/non-metallic character. (g) Predict the properties of elements in Group I, VII and the transition elements using the Periodic Table. Group I (h) Describe lithium, sodium and potassium in Group I (the alkali metals) as a collection of relatively soft, low density metal showing a trend in melting point and in their reaction with water. Group VII (i) Describe chlorine, bromine and iodine in Group VII (the halogens) as a collection of diatomic non-metal showing a trend in colour, state and their displacement reaction with solution of other halide ions. 31 2 Suggested Activities Resources Activity 13.1 Experiment: To show the reactivity of group I metals with water. http://www.chemi stry.co.nz/mandel eev.htm http://www.period ictable.com/page s/AAE_History.ht ml http://www.upei.c a/~physics/p221/ pro00/periodicTbl e/page2.html http://chemlab.pc. maricopa.edu/per iodic/foldedtable. html http://webelement s.com www.chemicalele ments.com http://pearl1.lanl. gov/periodic http://www.wou.e du/las/physci/ch4 12/alttable.htm http://upei.ca/~ph ysics/p221/pro00/ periodicTble/pag e4.html http://chemicalele ments.com/group s/alkali.html Topic / Sub-topic Group O – Noble gases No. of Weeks Lesson Objectives (j) Describe the elements in Group 0 (the noble gases) as a collection of monatomic elements that are chemically unreactive and hence important in providing an inert atmosphere, e.g. argon and neon in light bulb, helium in balloons; argon in the manufacture of steel. (k) Describe the lack of reactivity of the noble gases in term of their electronic structure. Transition elements (l) Describe the central block of elements (transition metals) are metal having high melting points, high density, variable oxidation state and forming coloured compounds. (m) State the use of these elements and /or their compounds as catalyst, e.g. iron in the Haber process; vanadium(V) oxide in the Contact process; nickel in the hydrogenation of alkenes, and how catalyst are used in industry to lower energy demands and hence are economically advantageous and help to conserve energy sources. 32 Suggested Activities Resources Activity 13.3 Demonstration: To show coloured solution of transition metals. http://www.chemical elements.com/grou ps/halogens.html http://www.warpoetr y.co.uk/owen1.html http://barney.gonza ga.edu/~bpiermat/p oem/DulceetDecoru mEst.html TOPIC 14: ENERGY FROM CHEMICALS Duration: 3 weeks Prior Knowledge: Topic 4 – Chemical Bonding, Topic 5 – Chemical Formulae, Topic 7 – Stoichiometry and Mole Concepts Links to: Topic 15 – Electrolysis, Topic 20 – Organic Chemistry, Biology – Plant Nutrition Keywords: exothermic, endothermic, energy profile diagram, enthalpy changes, activation energy, bond breaking, bond making, fuel, Photosynthesis, heat of combustion, heat of neutralisation, heat of solution. Learning outcomes: Students should be able to: OR describe the meaning of the terms exothermic and endothermic. draw the energy profile diagram for exothermic reaction. draw the energy profile diagram for endothermic reaction. state what is meant by H in a reaction use the formulae below (also by referring to the energy profile diagrams) to determine whether a reaction is exothermic or endothermic: ΔH Ein - E out , where E in is energy taken in (absorbed) in the reaction which is endothermic. E out is energy given out (released) in the reaction which is exothermic. determine that the reaction is endothermic if E in is bigger than E out , ( H positive), and exothermic if E in is smaller than E out , ( H negative). ΔH E f - E i , where E f is the final energy level (products) E i is the initial energy level (reactants) 33 determine that the reaction is endothermic if E f bigger than E i , and exothermic is E f is smaller than E i . state that bond breaking is endothermic because heat energy is absorbed. state that bond forming/making is exothermic because heat energy is released. explain in term of change in heat energy of bond breaking and bond forming/making exothermic or endothermic reactions. calculate heat of reaction for a given reaction with bond energies. state that combustion is an example of exothermic reaction. state that hydrogen is needed to generate electricity in a fuel cell, together with oxygen. discuss the production of electrical energy from simple cell, with respect to reactivity series. Topic / Sub-topic TOPIC 14 Energy From Chemicals Exothermic reaction Endothermic reaction Energy profile diagram Bond energy Enthalpy change No. of Weeks Lesson Objectives Activity 14.1 Practical: To find ΔH using 0.1M HCl and 0.1M NaOH solutions. Students should be able to: (a) Describe the meaning of enthalpy change in term of exothermic ( H negative) and endothermic ( H positive) reactions. Activity 14.2 Demonstration: To investigate heat of solution of salts. (b) Represent energy changes by energy profile diagrams, including reaction enthalpy changes and activation energies. Activity 14.3 Experiment: To set up Daniel cell. (c) Describe bond breaking as an endothermic process and bon making as an exothermic process. (d) Explain overall enthalpy changes in term of the energy changes associated with the breaking and making covalent bonds. (e) Describe combustion of fuels as exothermic, e.g. wood; coal; oil; natural gas; hydrogen. (f) Describe hydrogen, derived from water or hydrocarbons, as a potential fuel for use in future, reacting with oxygen to generate electricity directly in a fuel cell (details of the construction and operation of a fuel cell are not required) and discuss the advantages and disadvantages of this. (g) Name natural gas, mainly methane, and petroleum as a sources of energy. (h) Describe photosynthesis as the reaction between carbon dioxide and water in the presence of chlorophyll, using sunlight (energy) to produce glucose and explain how this can provide a renewable energy source. Simple cell (i) Describe the production of electrical energy from simple cell (i.e. two electrodes in an electrolyte) linked to the reactivity series. 34 Suggested Activities 3 Resources TOPIC 15: ELECTROLYSIS Duration: 4 weeks Prior Knowledge: Topic 4 – Chemical bonding, ionic equations Links to: Topic 5 – Chemical formulae, Topic 18 - Redox Keywords: electrode, anode, cathode, discharged, electrochemical series, electrolytic cell, anion, anode, cation, electrochemical series, electrolytic cell, dry cell, electrolytes, electroplating, electrode reaction, inert electrode, non-electrolyte, reactive electrode, refine, selective discharged, molten, aqueous, concentrated. Misconception: There is a tendency that students are not really able to distinguish between electrolytic cell from simple cell (chemical cell). Learning outcomes: Students should be able to: define electrolysis, electrodes and electrolytes. draw and label diagram of an electrolytic cell. give examples of some electrolytes and states the ions for each. state the movement and direction of anions, cations and electrons in electrolytic cell. describe the observations and write electrode reactions that occur at the anode and cathode during electrolysis. describe the change (if any) in the electrolyte during electrolysis. state that aqueous electrolytes are the mixture of an ionic solid dissolved in water. state that the selective discharged of ions is based on the following factors: Position of ions in the electrochemical series. Concentration of ions Nature of electrode predict the ions to be discharged and the products formed in electrolysis of given electrolytes. state that some ions in aqueous solution are not easily discharged even though they are present in high concentration. example: Anions: F - , SO 2, NO -3 , and CO 24 3 35 Cations: K , Na , Ca 2 , Mg 2 , and Al 3 describe the extraction of reactive metals by electrolytic process, example extraction of aluminium. explain the production of chemical during electrolysis such as chlorine and sodium chloride from concentrated sodium chloride solution. describe electroplating of metals such as copper using aqueous copper(II) suphate. state the importance of electroplating of metal. Topic / Sub-topic TOPIC 15 Electrolysis Introduction to electrolysis No. of Weeks Lesson Objectives Students should be able to: (a) Describe electrolysis as the conduction of electricity by an ionic compound (an electrolyte, when molten or dissolved in water, leading to the decomposition of the electrolyte. Electrolysis of molten electrolytes (c) Describe the mobility of ions present and the electrode products, the electrolysis of molten lead bromide, using inert electrodes. 4 Electrolysis of aqueous electrolytes (e) Apply idea of selective discharge (linked to the reactivity series for cations) to deduce the electrolysis of concentrated aqueous sodium chloride, aqueous copper(II) sulphate and dilute sulphuric acid using inert electrodes. (f) Predict the likely products of the electrolysis of an aqueous electrolyte, given relevant information. (g) Construct ionic equations for the reactions occurring at the electrodes during the electrolysis of the substances mentioned in the syllabus. 36 Activity 15.1 Demonstration on electrolysis of molten lead(II) bromide. http://www.corrosion doctors.org/Electro winning/Copper.htm http://www.chs.edu. sg/~limth/lessons/2 002/Electrolysis/rea ctive_electrodes.ht m http://www.extremet ech.com/article2/0,1 697,1155265,00.as p http://www.ce.org/Pr ess/CEA_Pubs/942. asp http://.www.energize r.com/learning/histor yofbatteries.asp http://www.buchman n.ca/chap1page3.asp http://www.corrosion doctors.org/Biogrpa hies/VoltaBio.htm http://www.howstuff works.com/battery2. htm Activity 15.3 Demonstration on electrolysis of concentrated sodium chloride solution. (d) Predict the likely product of the electrolysis of a molten binary compound. Resources Activity 15.2 Demonstration on electrolysis of dilute sodium chloride solution. (b) Describe electrolysis as evidence for the existence of ions which are held in a lattice when solid but which are free to move when molten or in solution. Suggested Activities Topic / Sub-topic Electrolysis in industry No. of Weeks Lesson Objectives (h) Describe the electrolysis of aqueous copper(II) sulphate with copper electrodes as means of purifying copper. (i) (j) Describe the electroplating of metals, e.g. copper plating, and recall one use of electroplating. Describe the electrolysis of purified aluminium oxide dissolved in molten cryolite as the method of extraction of aluminium (starting materials and essential conditions, including identity of electrodes should be given together with equation for the electrode reactions but no technical details or diagrams are required). (k) Explain the apparent lack of reactivity of aluminium. (l) State the uses of aluminium and relate the uses to the properties of this metal and its alloys, e.g. the manufacture of aircraft; food containers; electrical cables. 37 Suggested Activities Resources Activity 15.4 Demonstration on electrolysis sulphate using carbon electrodes. of copper(II) Activity 15.5 Demonstration on electrolysis sulphate using copper electrodes. of copper(II) Activity 15.6 Demonstration on electroplating of spatula with copper. TOPIC 16: SPEED OF REACTION Duration: 4 weeks Prior Knowledge: Relate gradient from graph (volume against time) to speed, interpret graphs given (from physics and maths) Links to: Topic 2 – Kinetic Particle Theory, Topic 6 – Types of Common Chemical Reactions, Topic 7 – Stoichiometry and Mole Concept, Topic 14 – Energy from Chemicals Keywords: speed of reaction, gradient, catalyst, temperature, particle size, concentration, pressure, activation energy, measurable speed, non-measurable speed. Misconception: Substances having bigger particle size are misconceived as having larger total surface area. Volumes of solutions are misconceived to be a factor of rate of reaction. Learning outcomes: Students should be able to: explain how pathways with lower activation energies account for the increase in speeds of reactions. relate the height of the Activation Energy to the speed of reaction. state that transition elements and their compounds act as catalyst in a range of industrial processes and that the enzymes are biological catalyst. give examples of catalysts and their related industrial uses. relate the speed of reaction to changes in temperature, concentration, particle size and pressure. suggest suitable method for investigating the effect of a given variable on the speed of a reaction. 38 describe with the aid of diagrams how to measure the speed of reaction between: (a) hydrochloric acid and sodium thiosulphate based on the speed of formation of sulphur. (b) calcium carbonate and hydrochloric acid based on the rate of formation of carbon dioxide interpret data obtained from experiments concerned with speed of reaction. interpret the speed from the data and the graph profile. Relate the gradient to the speed of the reaction. When the gradient becomes zero means that the reaction has completed. Topic / Sub-topic TOPIC 16 Speed of Reactions No. of Weeks Lesson Objectives Students should be able to: (a) Describe the effect of concentration, pressure, particle size and temperature on the speeds of reactions and explain the effect in term of collisions between reacting particles. (d) State that transition elements and their compounds act as catalyst in a range of industrial processes and that enzymes are biological catalyst. (e) Suggest suitable method for investigating the effect of a given variable on the speed of a reaction. (f) Interpret data obtained from experiments concerned with speed of reaction 39 Resources Activity 16.1 Experiment: To show the effect of concentration on the speed of reaction. http://www.chem4ki ds.com/files/react_r ates.html http://www.scijournal.org/index.ph p?template_type=re port&id=46&htm=re ports/vol1no1/v1n1k 44.htm&link=reports /home.php http://youth.net/nsrc /sci/sci035.html#anc hor1124013 Activity 16.2 Experiment: To show the effect of temperature on the speed of reaction. (b) Define the term catalyst and describe the effect of catalyst (including enzymes) on the speeds of reactions. (c) Explain how pathways with lower activation energies account for the increase in speeds of reactions. Suggested Activities 4 Activity 16.3 Experiment: To show the effect of particle size using calcium carbonate (lump and powder) with hydrochloric acid. Activity 16.4 Experiment: To show the decomposition of hydrogen peroxide using manganese(IV) oxide. TOPIC 17: REVERSIBLE REACTIONS Duration: 3 weeks Prior Knowledge: Topic 16 – Speed of Reaction, Topic 14 – Energy from Chemicals. Links to: Topic 6 – Types of Common Chemical Reactions, Topic 7 – Stoichiometry and Mole Concept, Topic 9 – Acids, Bases and Neutralisation, Topic 10 - Salt Keywords: Le Chatelier’s principle, dynamic equilibrium, backward reaction, forward reaction, (variables affecting shift in reaction- pressure, concentration, temperature), speed of reaction, Haber process, Contact process. Misconception: Increase in temperature is misconceived to shift the equilibrium forward irrespective of whether it is exothermic or endothermic; Increase in pressure for gaseous reactants is misconceived as shift in the forward direction irrespective whether there is a difference in the volume of the products. Equilibrium in reversible reaction must be seen as dynamic not static. Learning outcomes: Students should be able to: state that interconversion of state of water is a reversible process. state some reversible reactions in the lab, for example heating hydrated copper (II) sulphate, converting potassium chromate (VI) to potassium dichromate (VI) and vice versa by the addition of acid and alkali. apply Le Chatelier’s Principle to predict the equilibrium shift when variables are changed. state what is meant by dynamic equilibrium. Relate the equilibrium shift to changes in temperature, concentration and pressure. state the conditions for Haber process. 40 predict what will happen to the speed of reaction and shift of equilibrium when any of the variables (temperature, concentration and pressure) are changed. state the reversible reactions involved in Contact process state the uses of sulphur dioxide and sulphuric acid. state the functions of the essential N, P, K elements for plants. calculate % content of N, P, K in fertilizers. describe the effects of eutrophication to the eco-system. relate how adding ammonium fertilizers and liming can lead to unwanted loss of ammonia. Topic / Sub-topic TOPIC 17 Reversible Reaction Le Chatelier’s principle No. of Weeks Lesson Objectives Activity 17.1 Practical: To show reversible reactions. Students should be able to: (a) State that some chemical reactions are reversible. (b) Understand Le Chatelier’s principle. (c) Describe the idea that some chemical reactions can be reversed by changing the reaction conditions. (d) Describe the idea that some reversible reactions can reach dynamic equilibrium and predict the effect of changing the conditions. Haber process (e) Describe the use of nitrogen, from air, and hydrogen, from cracking oil, in the manufacture of ammonia. (f) Suggested Activities Describe the essential conditions for the manufacture of ammonia by the Haber process. (g) Describe the use of nitrogenous fertilisers in promoting plant growth and crop yield. (h) Compare nitrogen content of salts used for fertilisers by calculating percentage masses. (i) Describe eutrophication and water pollution problems caused by nitrates leaching from farm land and explain why the high solubility of nitrates increases these problems. (j) Describe the displacement of ammonia from its salts and explain why adding calcium hydroxide to soil can cause the loss of nitrogen from added nitrogenous fertiliser. 41 3 Resources Topic / Sub-topic Contact process No. of Weeks Lesson Objectives (k) Describe the manufacture of sulphuric acid from the raw material sulphur, air and water in the Contact process. (l) State the use of sulphur dioxide as a bleach, in the manufacture of wood pulp for paper and as a food preservative (by killing bacteria) (m) State the use of sulphuric acid in the manufacture of detergents and fertilisers; and as a battery acid. 42 Suggested Activities Activity 17.2 Experiment: To prepare fertiliser using nitric acid (the manufacture of fertilizer from ammonia). Resources TOPIC 18: REDOX Duration: 4 weeks Prior Knowledge: Topic 3 – Atomic Structure, Topic 4 – Chemical Formulae Links to: Topic 6 – Types of Common Chemical Reaction, Topic 12 – Metals and Extraction, Topic 16 – Electrolysis Keywords: Oxidation, reduction, oxidising agent, reducing agent, oxidation number/state. Misconception: 1. Oxidation or reduction is NOT a reaction that is all by itself. For example, the burning of magnesium in the air is not oxidation as what most people say it. It is redox. 2. A substance can be an oxidising agent in one reaction can be a reducing agent in another. For example, hydrogen peroxide, a common oxidising agent, is not necessarily an oxidising agent all the time. Learning outcomes: Students should be able to: interpret half equations as oxidation or reduction by the loss/gain of electrons. calculate the oxidation number/state of elements in binary and polyatomic compounds. 43 identify changes in oxidation number/state of elements involved in redox reaction. state the colour changes of oxidising and reducing agents in redox reactions. identify oxidising and reducing agents from symbol equation of a redox reaction. Topic / Sub-topic TOPIC 18 Redox No. of Weeks Lesson Objectives Students should be able to: (a) Define oxidation and reduction (redox) in terms of oxygen/hydrogen gain/loss. (b) Define redox in term of electron transfer and changes in oxidation states. (c) Identify redox reactions in terms of oxygen/hydrogen, and/or electron, gain/loss, and/or changes in oxidation state. (d) Describe the use of aqueous potassium iodide, and acidified potassium manganate(VII) and acidified potassium dichromate(VI) in testing for oxidising and reducing agents from the resulting colour changes. 44 4 Suggested Activities Resources Activity 18.1 Demonstration: To show the colour changes in oxidising agents – acidified potassium manganate(VII) and acidified potassium dichromate. http://www.chemistr y.co.nz/redox_oxi_a a.htm http://www.chemistr y.co.nz/redox_test.h tm Activity 18.2 Demonstration: To show the colour change of iodide ion in redox reaction. TOPIC 19: ATMOSPHERE AND ENVIRONMENT Duration: 2 weeks Prior Knowledge: Gases in the air and composition, pollutant gases, complete and incomplete combustion, bacterial decay of vegetable matter, industrial wastes, the formation of acid rain, depletion of ozone layer. Links to: Biology – Effects of man on the ecosystem. Keywords: Gaseous pollutants, effluent, complete and incomplete combustion, catalytic converter, ozone layer, greenhouse effect, eutrophication, chlorofluorocarbon. Misconception: 1. Carbon dioxide, a waste product of respiration, is not a pollutant as some people may have thought so. 2. Ozone is a harmful gas though its presence in the upper atmosphere is useful in screening of the ultraviolet radiation from the sun. Learning outcomes: Students should be able to: name some common gaseous pollutants in the air and their sources. explain the effects of gaseous pollutants on health and environment. describe the formation of acid rain. describe the formation of carbon monoxide gas from incomplete combustion of fuels. explain the need for catalytic converters in cars to reduce air pollution. 45 explain the importance of ozone layer in the atmosphere. state some greenhouse gases and how they cause global warming. explain the effect of effluents on aquatic life. describe the use of chemical fertilisers in farming as an environmental hazard. Topic / Sub-topic TOPIC 19 Atmosphere and Environment Air No. of Weeks Lesson Objectives Suggested Activities Activity 19.1 Students’ research and presentation. Students should be able to: (a) Describe the volume composition of gases present in dry air as 79% nitrogen, 20% oxygen and the remainder being noble gases (with argon as the main constituent) and carbon dioxide. (b) Describe the separation of oxygen, nitrogen and the noble gases from liquid air by fractional distillation. (c) State the use of oxygen (e.g. making steel; oxygen tents in hospitals; together with acetylene, in welding). (d) Name common atmospheric pollutants (e.g. carbon monoxide; methane’ nitrogen oxides ( NO and NO 2 ); ozone; sulphur dioxide; unburned hydrocarbons). (e) State the source of these pollutants as: 2 (i) Carbon monoxide from the incomplete combustion of carbon-containing substances. (ii) Methane from bacterial decay of vegetable matter. (iii) Nitrogen oxides from lightning activity and internal combustion engines. (iv) Ozone from photochemical reactions responsible for the formation of photochemical smog. (v) Sulphur dioxide from volcanoes and combustion of fossil fuels. (vi) Unburned hydrocarbons combustion engines. from internal 46 Resources http://www.scijounal.org/index.php ?template_type=rep ort&id=28&htm=repr ts/vol3no1/v3n1k43. html&link=reports/h ome.php http://youth.net/nsrc /sci/sc047.html#anc hor1415078 http://www.nea.gov. sg/psi/ http://epa.gov/acodr ain/index.html http://www.geocities .com/whatsacidrain/ http://www.angelfire. com/ks/boredwalk/ http://www.sciences horts.com/articles/a cid%20Rain.htm http://www.madison. k12.wi.us/stugeon/a cfacts.htm http://www.epa.gov/ globalwarming http://youth.net/nsrc /sci/sci023.html#anc hor1264372 http://youth.net/nsrc /sci/sci023.html#anc hor1266081 Topic / Sub-topic No. of Weeks Lesson Objectives (f) Describe the reaction used in possible solutions to the problems arising from some of the pollutants named in (d). (i) The redox reactions in catalytic converters to remove combustion pollutants. (ii) The use of calcium carbonate to reduce the effect of ‘acid rain’ and flu gas desulphurisation. (g) Discuss some of the effects of these pollutants on health and on the environment. (i) The poisonous nature of carbon monoxide. (ii) The role of nitrogen dioxide and sulphur dioxide in the formation of ‘acid rain’ and its effect on respiration and building. (h) Discuss the importance of the ozone layer and the problem involved with the depletion of ozone by reaction with chlorine containing compounds, chlorofluorocarbons (CFCs). (i) (j) Describe the carbon cycle in simple terms, to include: (i) The processes of combustion, respiration and photosynthesis. (ii) How carbon cycle regulate the amount of carbon dioxide in the atmosphere. State that carbon dioxide and methane are greenhouse gases and may contribute to global warming, give the sources of these gases and discuss the possible consequences of an increase in global warming. 47 Suggested Activities Resources Topic / Sub-topic Water No. of Weeks Lesson Objectives (k) State that water from natural sources contains a variety of dissolved substances. (l) (i) Naturally occurring (mineral salts; oxygen; organic matter). (ii) Pollutant (metal compounds; sewage; nitrate from fertilisers; phosphates from fertilisers and detergents; harmful microbes) Discuss the environmental effect of the dissolved substances named in (a) (i) Beneficial, e.g. oxygen and mineral salts for aquatic life. (ii) Pollutant, e.g. hazard to health; eutrophication. (m) Outline the purification of water supply in term of: (i) Filtration to remove solids. (ii) Use of carbon to remove taste and odours. (iii) Chlorination to disinfect the water. (n) State that seawater can be converted into drinkable water by desalination. 48 Suggested Activities Activity 19.2 Demonstration: To show test for water using blue cobalt chloride paper and anhydrous copper(II) sulphate. Activity 19.3 Demonstration on water treatment using alum (pond water) Activity 19.4 Enrichment – visit to water treatment plant. Resources TOPIC 20a: ORGANIC CHEMISTRY – PETROLEUM (HYDROCARBON) Duration: 1 week Prior Knowledge: Topic 2 – Kinetic Particle Theory, Topic 8 – Experimental Chemistry, Topic 5 – Chemical Formulae Links to: Topic 4 – Chemical Bonding Keywords: Petroleum, natural gas, hydrocarbon, petroleum fractions: petrol, naphtha, paraffin, diesel, lubricating oil, bitumen, complete and incomplete combustion Misconception: There is a tendency to misconceive petroleum as petrol. Learning outcomes: Students should be able to: name sources of fuels other than petroleum. define petroleum. describe the fractional distillation of petroleum. explain how fractionating columns separate the petroleum fractions. name six petroleum fractions. state the uses for each fraction in the petroleum. 49 describe the changes in physical properties (melting point, boiling point, viscosity and flammability) of the fractions from top to bottom of the fractionating column. name the products for the complete combustion of hydrocarbon fuels. name the products for the incomplete combustion of hydrocarbon fuels. Topic / Sub-topic TOPIC 20 Organic Chemistry Introduction Hydrocarbon Petroleum No. of Weeks Lesson Objectives Students should be able to: (a) Describe petroleum as a mixture of hydrocarbons and its separation into useful fraction by fractional distillation. (b) Name the following fractions and state their uses: (i) Petrol (gasoline) as fuel in cars (ii) Naphtha as feedstock for chemical industry (iii) Paraffin (kerosene) as a fuel for heating and cooking and for aircraft engines. (iv) Diesel as a fuel for diesel engines (v) Lubricating oils as lubricants and as a source of polishes and waxes. (vi) Bitumen for making road surfaces. (c) State that the naphtha fraction from crude oil is the main source of hydrocarbons used as feedstock for the production of a wide range of organic compounds. (d) Describe the issues relating to the competing uses of oil as an energy source and as chemical feedstock. 50 1 Suggested Activities Resources TOPIC 20b: ORGANIC CHEMISTRY – ALKANES Duration: 1 week Prior Knowledge: Topic 4 – Chemical Bonding, Topic 5 – Chemical Formulae, Topic 6 – Types of Common Chemical Reactions Links to: Topic 20a – Petroleum (hydrocarbon) Keywords: homologous series, general formula, unbranched alkanes, branched alkanes, molecular formula, structural formula, saturated, viscosity, flammability, isomerism, combustion, substitution. Learning outcomes: Students should be able to: define homologous series. describe the alkanes as the homologous series of saturated hydrocarbons with the general formula C n H 2n2 . deduce the molecular formula of the alkanes up to 6 carbon atoms and name them. 51 draw the structural formulae of the unbranched alkanes up to 6 carbon atoms. draw the structural formulae of the branched alkanes up to 6 carbon atoms. define saturated hydrocarbon. describe the chemical properties of alkanes. define isomerism and identify isomers. Topic / Sub-topic Alkanes No. of Weeks Lesson Objectives Students should be able to: (a) Describe a homologous series as a group of compound with a general formula, similar chemical properties and showing a gradation in physical properties as a result of increase in the size and mass of the molecules, e.g. melting and boiling points, viscosity; flammability. (b) Describe the alkanes as an homologous series of saturated hydrocarbons with the general formula C n H2 n 2 . (c) Draw the structures of branched and unbranched alkanes, C1 to C4 and name the unbranched alkanes, methane to butane. (d) Define isomerism and identify isomers (e) Describe the properties of alkanes (exemplified by methane) as generally unreactive except in terms burning and substitution by chlorine. 52 Suggested Activities Resources Activity 20.1 Demonstration: To show incomplete combustion of hydrocarbon. http://www.energyq uest.ca.gov/story/ch apter08.html http://www.kcpc.usy d.edu.au/discovery/ 9.2.1/index.html http://www.pafko.co m/history//h_petro.h tml http://www.pafko.co m/history//h_refine. html http://www.howstuff works.com/newsitem10.htm http://inventors.abo ut.com/library/weekl y/aa090299.htm Activity 20.2 Constructing molecules of organic compounds using models. 1 TOPIC 20c: ORGANIC CHEMISTRY – ALKENES Duration: 1 week Prior Knowledge: Topic 4 – Chemical Bonding, Topic 5 – Chemical Formulae, Topic 6 – Types of Common Chemical Reactions Links to: Topic 20a – Petroleum (hydrocarbon), Topic 20b - Alkanes Keywords: unsaturated, functional group, addition, hydrogenation, hydration, halogenation, cracking, polymerization, polymer, monomer, polyunsaturated. Learning outcomes: Students should be able to: describe alkenes as the homologous series of unsaturated hydrocarbons with the general formula C n H 2n . state the functional group of alkenes. deduce the molecular formula of the alkenes up to 6 carbon atoms and name them. draw the structural formulae of the unbranched alkenes up to 6 carbon atoms. draw the structural formulae of the branched alkenes up to 6 carbon atoms. define unsaturated hydrocarbon. State the combustion of alkenes, including equation and conditions (if any). state the addition of alkenes with hydrogen, steam, and halogen, including equation and conditions (if any). 53 state the polymerization of alkenes or alkene derivatives, including equation and condition. describe the manufacture of alkenes and hydrogen by cracking of big alkanes. state the importance of cracking process describe the use of aqueous bromine to distinguish saturated hydrocarbons from unsaturated hydrocarbons. describe the difference between saturated and unsaturated compounds from their molecular structures. state the meaning of polyunsaturated when applied to food products, e.g. vegetable oil. describe the manufacture of margarine by catalytic hydrogenation of vegetable oil. Topic / Sub-topic Alkenes No. of Weeks Lesson Objectives Activity 20.3 Demonstration: To test for alkenes with bromine. Students should be able to: (a) Describe the alkenes as a homologous series of unsaturated hydrocarbons with the general formula C n H2 n . (b) Draw the structure of branched and unbranched alkenes, C2 to C4 and name the unbranched alkenes, ethene to butene. (c) Describe the manufacture of alkenes and hydrogen by cracking hydrocarbons and recognise that cracking is essential to match the demand for fractions containing smaller molecules from the refinery process. (d) Describe the properties of alkenes in terms of combustion, polymerisation and their addition reactions with bromine, steam and hydrogen. (e) Describe the difference between saturated and unsaturated hydrocarbons from their molecular formula and by using aqueous bromine. (f) Suggested Activities Describe the meaning of polyunsaturated when applied to food product. (g) Describe the manufacture of margarine by the addition of hydrogen to unsaturated vegetable oils to form a solid product. 54 1 Resources http://www.automoti vetechnology.com/proj ects/p2000/index,ht ml#p20001 http://energy.saving. nu/biomass/carsbiof uel.shtml http://www.nesea.or g/greecarclub/factsh eets_ethanol.pdf http://nobelprize.org /educational_games /chemistry/conductiv e_polymers/index.ht ml TOPIC 20d: ORGANIC CHEMISTRY – ALCOHOLS Duration: 1 week Prior Knowledge: Topic 4 – Chemical Bonding, Topic 5 – Chemical Formulae, Topic 6 – Types of Common Chemical Reactions, Topic 18 - Redox Links to: Topic 20a – Petroleum (hydrocarbon), Topic 20b – Alkanes, Topic 20c – Alkenes Keywords: oxidation, fermentation, functional group, fluidity, flammability, hydroxyl group, hydration, combustion, dehydration Misconception: Students misconceived that all alcohols are consumable when in fact ethanol is the only consumable alcohol. Learning outcomes: Students should be able to: describe alcohol as the homologous series containing the –OH functional group. describe alcohol as the homologous series with the general formula C n H 2n1OH give the name of the first six members of the alcohols. draw the structural formulae of the unbranched alcohol up to 6 carbon atoms. 55 describe the preparation of ethanol by catalysed addition of steam to ethene and by fermentation of glucose. describe the chemical reactions of alcohol such as combustion, dehydration and oxidation. state some uses of ethanol. Topic / Sub-topic Alcohols No. of Weeks Lesson Objectives Activity 20.4 Demonstration: To compare the flammability and the colour of the flames produced by different alcohols and to show the variation of physical properties of the first four members of alcohol. Students should be able to: (a) Describe the alcohols as a homologous series containing the OH group. (b) Draw the structures of alcohols, C1 to C4 and name the unbranched alcohols, methanol to butanol. (c) Describe the properties of alcohols in terms of combustion and oxidation to carboxylic acids. (d) Describe the formation of ethanol by the catalysed addition of steam to ethene and by fermentation of glucose. (e) State some uses of ethanol, e.g. as a solvent, as a renewable fuel; as a constituent of alcoholic beverages. 56 Suggested Activities Activity 20.5 Demonstration: To compare fluidity of the alcohols. 1 Resources TOPIC 20e: ORGANIC CHEMISTRY – ORGANIC ACIDS (CARBOXYLIC ACIDS) Duration: 1 week Prior Knowledge: Topic 9 – Acids, Bases and Neutralisation Links to: Topic 20a – Petroleum (hydrocarbon), Topic 20b - Alkanes, Topic 20c - Alkenes, Topic 20d – Alcohols Keywords: weak acid, oxidation, esterification, carboxyl group Misconception: This is one organic compound or covalent compound that ionises; therefore it is also an ionic compound. Learning outcomes: Students should be able to: describe carboxylic acid as the homologous series containing COOH group. give the name of the first six members of the carboxylic acids. draw the structural formulae of the unbranched carboxylic acids up to 6 carbon atoms. state with reasons why carboxylic acid is a weak acid. state the reactions between carboxylic acid with carbonates. state the reactions between carboxylic acid with bases. 57 state the reactions between carboxylic acid with some metals. state the physical properties of carboxylic acid. describe the formation of ethanoic acid by the oxidation of ethanol. state the esterification between ethanoic acid and ethanol including equation and condition (if any). state commercial uses of ester. Topic / Sub-topic Carboxylic Acids No. of Weeks Lesson Objectives Activity 20.6 Demonstration: To show oxidation of ethanol to ethanoic acid. Students should be able to: (a) Describe the carboxylic acids as a homologous series containing COOH group. Activity 20.7 Demonstration: To show the acidic properties of carboxylic acid. (b) Draw the structures of carboxylic acids, C1 to C4 and name the unbranched acids, methanoic to butanoic acids. (c) Describe the carboxylic acids as weak acids, reacting with carbonates, bases and some metals. (d) Describe the formation of ethanoic acid by oxidation of ethanol by atmospheric oxygen or acidified potassium dichromate(VI). (e) Describe the reaction of ethanoic acid with ethanol to form ester, ethyl ethanoate. (f) State some commercial uses of ester, e.g. perfumes; flavouring; solvents. 58 Suggested Activities 1 Resources TOPIC 20f: ORGANIC CHEMISTRY – MACROMOLECULES (POLYMERS) Duration: 1 weeks Links to: Topic 20a – Petroleum (hydrocarbon), Topic 20b - Alkanes, Topic 20c - Alkenes, Topic 20d - Alcohols, Topic 20e – Carboxylic acids, Biology – starch, protein, and fat. Keywords: monomers, repeating units, plastic, addition polymer, condensation polymer, synthetic polymer, natural polymer, amide linkage, ester linkage, non-biodegradable, hydrolysis. Learning outcomes: Students should be able to: describe the term macromolecule. describe the formation of addition polymers such as poly(ethene), poly(chloroethene), poly(styrene) and poly(tetrafluoroethene). draw the structures of the above polymers. deduce the structure of monomers from given addition polymers. state the uses of the above polymers. define condensation polymerisation as exemplified by Terylene and nylon. show the joining of two different monomers in the formation of nylon and Terylene. given the structure of nylon and Terylene identify the monomers. name the linkages found in nylon and Terylene. 59 state some uses of nylon and Terylene. identify the types of polymer from the block representation. describe the pollution problems caused by plastics which are nonbiodegradable. describe starch, protein and fat as natural polymers and identify the monomers of each. state the linkages in proteins, fat and starch. describe the similarities and differences between the structure of nylon and protein and between Terylene and fats. describe the hydrolysis of proteins to amino acids and starch to simple sugars. Topic / Sub-topic Macromolecules - polymers No. of Weeks Lesson Objectives Students should be able to: (a) Describe macromolecules as a large molecules built up from small unit, different macromolecules having different unit and/or different linkages. (b) Describe the formation of poly(ethene) as an example of addition polymerisation of ethene as monomer. (c) State some uses of poly(ethene) as a typical plastic, e.g. plastic bags, cling film. (d) Deduce the structure of the polymer product from a given monomer and vice versa. (e) Describe nylon, a polyamide, and Terylene, a polyester, as condensation polymers (refer syllabus for the partial structures of nylon and Terylene). 1 (f) State some typical uses of man-made fibres such as nylon and Terylene, e.g. clothing, curtain materials; fishing line; parachutes; sleeping bags. (g) Describe the pollution problems caused by the disposal of non-biodegradable plastics. (h) Identify carbohydrates, proteins and fats as natural macromolecules. (i) Describe proteins as possessing the same amide linkages as nylon but different monomer units. (j) Describe fat as esters possessing the same linkages as Terylene but with different monomer units. (k) Describe hydrolysis of proteins to amino acids and carbohydrates (e.g. starch) to simple sugars. 60 Suggested Activities Resources