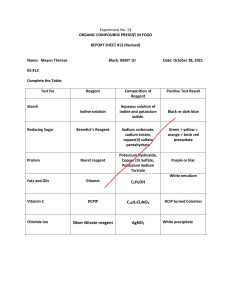
AP Chemistry Laboratory IV Report Precipitation of Barium Sulfate and Lead Chromate 02/16/2022 I. Objective: The objective of the laboratory is to process two different precipitation reactions: to precipitate barium sulfate (BaSO4) by mixing two solutions of barium chloride (BaCl2) and sodium sulfate (NaSO4) and to precipitate lead chromate (PbCrO4) by mixing two solutions of lead acetate trihydrate [Pb(COOCCH3)2·3(H2O)] and potassium dichromate (K2Cr2O7). II. Purpose: The purpose of the laboratory experiment is to learn precipitation reaction; understand the chemical equation used in precipitation; gain knowledge of laboratory experiments; understand the concept of stoichiometry; calculate both experimental yield and theoretical yield; calculate the percent yield. III. Theory: There are three common types of chemical reactions which are: oxidation-reduction, acid-base, and precipitation reactions. A precipitation reaction is a reaction that forms an insoluble substance when two solutions are mixed. To identify the solid produced after mixing two reactants, the following conditions should be satisfied. 1. When a solid compound is formed, the products must contain both anions and cations and eventually have a zero net charge. 2. When the equation is perfectly balanced, according to solubility rules, the product that will remain in the solution will be determined. For example, a mixture of K2CrO4 and Ba(NO3)2 in a complete ionic equation is represented as: + 2− 2𝐾 (𝑎𝑞) + 𝐶𝑟𝑂4 (𝑎𝑞) + 𝐵𝑎 2+ − (𝑎𝑞) + 2𝑁𝑂3 (𝑎𝑞) → 2𝐾𝑁𝑂3 + 𝐵𝑎𝐶𝑟𝑂4 According to the solubility rule presented below, the product that precipitates out in a solid form is determined. 1. Salts containing Group I elements (Li+, Na+, K+, Cs+, Rb+) and ammonium ion (NH4+) are also soluble. 2. Salts containing nitrate ion (NO3-) are generally soluble. 3. Salts containing Cl -, Br -, or I - are generally soluble. Important exceptions to this rule are halide salts of Ag+, Pb2+, and (Hg2)2+. 4. Most silver salts are insoluble. Exception for AgNO3 and Ag(C2H3O2). 5. Most sulfate salts are soluble. Exceptions to this rule include CaSO4, BaSO4, PbSO4, Ag2SO4 and SrSO4. 6. Most hydroxide salts are only slightly soluble. Hydroxide salts of Group I elements are soluble. Hydroxide salts of Group II elements (Ca, Sr, and Ba) are slightly soluble. Hydroxide salts of transition metals and Al3+ are insoluble. 7. Most sulfides of transition metals are highly insoluble, including CdS, FeS, ZnS, and Ag2S. Arsenic, antimony, bismuth, and lead sulfides are also insoluble. 8. Carbonates (CO3) are frequently insoluble. Group II carbonates (CaCO3, SrCO3, and BaCO3) are insoluble, as are FeCO3 and PbCO3. 9. Chromates (CrO4) are frequently insoluble. 10. Phosphates (PO4) such as Ca3(PO4)2 and Ag3PO4 are frequently insoluble. 11. Fluorides (F2) such as BaF2, MgF2, and PbF2 are frequently insoluble. According to the solubility rule number two and nine, the product will precipitate in solid form is easily concluded to be BaCrO4, and 2KNO3 will remain with water. IV . Procedure Materials ● Chemicals (BaCl2, NaSO4, Pb(COOCCH3)2·3H2O, K2Cr2O7) ● Beakers (100mL, 150mL) ● Balance ● Hot plate ● Oven ● Filter paper ● Funnel ● Ring stand 1. Balancing the equation. a. Balance both BaSO4 and PbCrO4 equation. 2. Preparing aqueous solutions of limiting reagents (BaCl2 / Pb(COOCCH3)2·3H2O). a. Measure the mass of about 1.00 g of limiting reagents using an analytical balance. i. Open one of the doors of the analytical balance and place the weighing boat on the middle of the balance and close the door. ii. After the weighing boat is weighted, set the balance to 0.00g. iii. Open one of the doors of the balance and add approximately 1.00g or slightly over 1.00g of reagent to the weighing boat using spatula. iv. Gently lose the door of the balance and record the weight of the reagent. b. Place a 150 mL beaker and fill with about 30 mL of deionized water. c. Put the reagent in the beaker and dissolve it until the solution is well saturated. 3. Preparing aqueous solutions of excess reagents (NaSO4 / K2Cr2O7). a. Calculate the amount of excess reagents needed to activate the entire limiting reagent. i. 𝑋𝑔 𝑙𝑖𝑚𝑖𝑡𝑖𝑛𝑔 𝑟𝑒𝑎𝑔𝑒𝑛𝑡 1 𝑎𝑚𝑢 𝑜𝑓 𝑒𝑥𝑐𝑒𝑠𝑠 𝑟𝑒𝑎𝑔𝑒𝑛𝑡 𝑍 𝑚𝑜𝑙𝑒 𝑒𝑥𝑐𝑒𝑠𝑠 𝑟𝑒𝑎𝑔𝑒𝑛𝑡 𝑥 𝑌 𝑚𝑜𝑙𝑒 𝑙𝑖𝑚𝑖𝑡𝑖𝑛𝑔 𝑟𝑒𝑎𝑔𝑒𝑛𝑡 𝑎𝑚𝑢 𝑜𝑓 𝑙𝑖𝑚𝑖𝑡𝑖𝑛𝑔 𝑟𝑒𝑎𝑔𝑒𝑛𝑡 x 𝑚𝑜𝑙𝑒 𝑟𝑎𝑡𝑖𝑜 𝑜𝑓 𝑒𝑥𝑐𝑒𝑠𝑠 𝑟𝑒𝑎𝑔𝑒𝑛𝑡 𝑚𝑜𝑙𝑒 𝑟𝑎𝑡𝑖𝑜 𝑜𝑓 𝑙𝑖𝑚𝑖𝑡𝑖𝑛𝑔 𝑟𝑒𝑎𝑔𝑒𝑛𝑡 x x 1.1 b. Measure the mass of excess reagent similar to the calculated value using an analytical balance. c. Place a 100 mL beaker and fill with about 30 mL of deionized water. d. Put the reagent in the beaker and dissolve it until the solution is well saturated. 4. Dissolving liming reagent and excess reagent. a. Place the beaker with limiting reagent on the hot plate. b. Pour the excess reagent solution to the limiting reagent solution. c. Wait until every reagent undergoes a precipitation reaction and forms a solid state at the bottom of the beaker. i. Make sure to check every liming reagent is perfectly reacted by adding several drops of excess reagent and check if any particles occur in the supernatant. 5. Decanting the dissolved solution. a. Put the filter paper in the oven and let it dry completely. b. Measure the mass of the filter paper before decanting the solution. i. Measure the time that the filter paper is exposed to the air to be exactly 30 seconds then read the number shown on the balance. c. Prepare the ring stand and put the funnel into one of the ring and put the 150 mL under the cannel. d. Put the filter paper in the cannel and pour deionized water on the wall of the funnel to make sure the filter paper is perfectly attached to the funnel without any gap. e. Carefully pour the solution through the funnel. f. Fill the beaker with deionized water and decant the solution several times. i. Make sure all the precipitates in the surface of the beaker are all washed out and also poured through the funnel. 6. Measuring the actual yield of precipitate a. Wait until every supernatant passes through the funnel and put the filter in the oven. i. The time that the filter paper is exposed to the air should be constant to be 30 seconds or the mass of the filter paper will be measured differently by different amounts of water molecules the filter paper absorbs. b. Subtract the experimental yield of the precipitate by subtracting the mass of filter paper to the mass of the filter paper after precipitation. 7. Calculating the percent yield. i. IV. | 𝐴𝑐𝑡𝑢𝑎𝑙 𝑦𝑖𝑒𝑙𝑑 | · 100 | 𝑇ℎ𝑒𝑜𝑟𝑒𝑡𝑖𝑐𝑙𝑎 𝑦𝑖𝑒𝑙𝑑 | Data and Calculations: ● Equation Experiment 1: Precipitation of BaSO4 𝐵𝑎𝐶𝑙2 (𝑎𝑞) + 𝑁𝑎2𝑆𝑂4 (𝑎𝑞) → 𝐵𝑎𝑆𝑂4 (𝑠) + 2𝑁𝑎𝐶𝑙 (𝑎𝑞) Experiment 2: Precipitation of PbCrO4 Equilibrium equation: Cr2O72- + OH-⇌ 2CrO42- +2H+ + 2− 2𝐾 + 𝑃𝑏(𝐶2𝐻3𝑂2)2 · 3𝐻2𝑂 + 𝐶𝑟𝑂4 ● → 𝑃𝑏𝐶𝑟𝑂4(𝑠) + 2𝐾𝑂2 𝐶𝐻3 (𝑎𝑞) Mass of reagents Experiment 1 Experiment 2 Limiting reagent 1.1021 1.0522 Theoretical yield of excess reagent 0.8270 0.4488 Experimental yield of excess reagent 0.89 0.50 ○ Stoichiometry of calculating excess reagent Experiment 1: 1..1021𝑔 𝐵𝑎𝐶𝑙2 1 · 1 𝑚𝑜𝑙 𝐵𝑎𝐶𝑙2 208.23 𝑔 · 1 𝑚𝑜𝑙 𝑁𝑎2𝑆𝑂4 · 1 𝑚𝑜𝑙 𝐵𝑎𝐶𝑙2 142.04 𝑔 1 𝑚𝑜𝑙 𝑁𝑎2𝑆𝑂4 · 1. 1 = 0. 0827 𝑔 Experiment 2: 1.0522𝑔 𝑃𝑏(𝐶2𝐻3𝑂2)2·3𝐻2𝑂 1 · 1 𝑚𝑜𝑙 379.33 𝑔 𝑃𝑏(𝐶2𝐻3𝑂2)2·3𝐻2𝑂 · 1 𝑚𝑜𝑙 𝐶𝑟𝑂4 2− 1 𝑚𝑜𝑙 𝑃𝑏(𝐶2𝐻3𝑂2)2·3𝐻2𝑂 · 115.99𝑔 1 𝑚𝑜𝑙 𝐶𝑟𝑂4 2− = 0. 4488 𝑔 ● Mass of products Experiment 1 Experiment 2 Filter paper before decanting 1.4222 1.3327 Filter paper after decanting 2.6557 2.2117 Product 1.2335 0.9790 ○ Stoichiometry of calculating the theoretical yield of product Experiment 1: 1.3635𝑔 𝐵𝑎𝐶𝑙2 1 · 1 𝑚𝑜𝑙 𝐵𝑎𝐶𝑙2 208.23 𝑔 · 1 𝑚𝑜𝑙 𝐵𝑎𝑆𝑂4 1 𝑚𝑜𝑙 𝐵𝑎𝐶𝑙2 · 233.39 𝑔 1 𝑚𝑜𝑙 𝐵𝑎𝑆𝑂4 = 1. 52825 𝑔 Experiment 2: 1.0522𝑔 𝑃𝑏(𝐶2𝐻3𝑂2)2·3𝐻2𝑂 1 = 0. 89648 𝑔 · 1𝑚𝑜𝑙 𝑃𝑏(𝐶2𝐻3𝑂2)2·3𝐻2𝑂 379.33 𝑔 · 1 𝑚𝑜𝑙 𝑃𝑏𝐶𝑟𝑂4 1 𝑚𝑜𝑙 𝑃𝑏(𝐶2𝐻3𝑂2)2·3𝐻2𝑂 · 323.19𝑔 1 𝑚𝑜𝑙 𝑙 𝑃𝑏𝐶𝑟𝑂4 · 1. 1 ● Percent Yield Experiment 1: 1.2335 1.5283 · 100 = 80. 713% Experiment 2: 0.9790 0.8965 · 100 = 1. 092% V. Results and Discussion: A precipitation reaction is a reaction that forms an insoluble substance when two solutions are mixed. In this lab, two experiments proceed and eventually precipitate two solids–BaSO4 and PbCrO4. Precipitation of BaSO4 and PbCrO4 was structuralized by following equations: 𝐵𝑎𝐶𝑙2 (𝑎𝑞) + 𝑁𝑎2𝑆𝑂4 (𝑎𝑞) → 𝐵𝑎𝑆𝑂4 (𝑠) + 2𝑁𝑎𝐶𝑙 (𝑎𝑞) + 2− 2𝐾 + 𝑃𝑏(𝐶2𝐻3𝑂2)2 · 3𝐻2𝑂 + 𝐶𝑟𝑂4 → 𝑃𝑏𝐶𝑟𝑂4(𝑠) + 2𝐾𝑂2 𝐶𝐻3 (𝑎𝑞) The mass of the limiting reagents were weighed by analytical balance and mixed with water. After calculating the amount of excess reagents using stoichiometry, excess reagents were also mixed with water. Two solutions were mixed to one on the hot plate and an evenly saturated solution was decanted through filter paper where the precipitate will remain. After drying the filter paper using an oven and the mass of the product was calculated by subtracting the mass of the filter paper from the mass of the filter paper and the precipitate. The mass of limiting reagent, BaCl2 and Pb(COOCCH3)2·3H2O were 1.1021 g and 1.0522 g, and both solutions were mixed with each excess reagent solution NaSO4 and K2Cr2O7. The mass of filter paper before decanting was 1.4222 g and 1.3327 g, and the mass of filter paper after decanting was 2.6557 g and 2.2117 g, thereby the experimental yield of the precipitates was calculated to be 1.2335 g and 0.9790 g respectively. The theoretical yield of the product was calculated using stoichiometry and came out to be 1.5283 g and 0.89648 g. The percent yield of BaSO4 was 80.713% and PbCr2O4 was 1.092%. The errors occurred in the lab were due to the remaining precipitate in the beaker and the remaining excess reagent in the filter because the filter was not fully washed by water. VI. Conclusion The experimental and theoretical yields of BaSO4 were 1.2335 g and 1,5283 g, having 80.713% of the percent yield. The experimental and theoretical yields of PbCr2O were 0.9790 g and 0.89648 g respectively, having 1.092% of the percent yield.