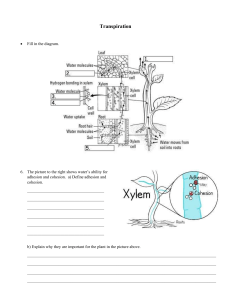
Basic Chemistry Notes Atoms are the __basic unit______ of matter and life. Atoms have ____3______ parts: __Protons_____, __Neutrons___, ___Electrons______ Elements are __pure____ substances made up of ___one__ type of atom. Matter is anything that has ___mass___ and takes up __space____. Matter is made up of ___elements___. Elements are represented by ____1 or 2_____ letter symbols. Compounds are substances formed by the __chemical__ combination of ___2 or more____ elements. Ionic bonds are __1 or more___ electrons __transferred___ from one atom to another. Covalent bonds are electrons __shared___ by atoms. Properties of Water Notes Water molecules are ___polar___ because there is an uneven distribution of ___electrons____ in the covalent bonds between the oxygen and hydrogen atoms. Oxygen is a more ___electronegative____ atom compared to hydrogen, so the electrons on the hydrogen atoms are pulled more towards the oxygen atom. As a result, the oxygen end of the molecule has a slight __negative_____ charge and the hydrogen end has a slight ___positive____ charge. Due to the polarity in water molecules, they form ___hydrogen bonds___ to other water molecules and other stuff. A single water molecule may be involved in as many as ____4_______ hydrogen bonds at a time. Water’s ability to form multiple hydrogen bonds is responsible for many of its __properties___. Properties of Water • _____Cohesion________________ • _________Solvent______________ • _______Adhesion______________ • ____Specific Heat Capacity_______ Adhesion • ____Adhesion____________ is an attraction between molecules of different substances. • Adhesion causes ____capillary action______ allowing water to rise in a narrow tube against the force of gravity. Cohesion • __Cohesion______________ is an attraction between molecules of the same substance. • Water's cohesion causes molecules on the surface of water to be drawn inward creating ___surface tension_____________. • This is why drops of water form __beads_____ on a smooth surface and why some ______insects___ can walk on a pond's surface. Other Properties of Water • High-specific heat: __The ability to raise the temperature of 1 gram of water by 1 Celsius__________ • High heat of vaporization: __The amount of energy to turn water in to a gas___________________ • The lower density of ice: ___the molecules of ice are farther apart because of the crystal structure____ • Universal solvent: __Water can dissolve most stuff but no everything _________ Carbon and Other Elements Carbon is one of the most ___important____ element of life. This is because ____complex___ molecules can be simply __formed__ by carbon and its ability to form ___4 covalent_____ bonds. _Hydrocarbons___ are long carbon chains with __hydrogen__ attached and are the ___basis_ for all macromolecules. Nitrogen is important for __Nucleic Acids____ and ___Proteins_____. Phosphorus is important for __Phospholipids____, ___Nucleic Acids_____, and ___Energy______. All __matter_____ must come from somewhere as it cannot be _created__ or _destroyed____. Living things try to increase their _surface area___ to ____volume____ ratio to try and absorb as much matter as possible.