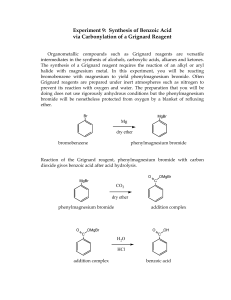
CHEM 3220 ORGANIC LABORATORY II EXPERIMENT HANDOUT UNIVERSITY OF NORTH TEXAS Week # 3 and 4 – Experiment 2: Grignard Synthesis of Benzoic acid from Phenylmagnesium bromide Additional Reading from the manual 6th Edition(pages) Chapter 38.1 7th Edition(pages) 495- 497 500-503 Please read the notes before proceeding further. Your TA will assign the bench and hood space during the first lab, will remain the same for the rest of the semester. Stay at your bench unless asked to start the experiment. Prelab is MANDATORY. Make corrections or edits to your procedure/protocol in the “Final Report” as per the experiment performed in the lab. Please watch the video before the experiment, the information from the video will be helpful while performing the experiment. Do not forget to carry safety goggles, dress code and foot protection. Prepare prelab and submit (online). Carry a copy of the prelab to the laboratory. Prelab should contain - Objective, Materials list (chemicals) Reference table, and procedure. In the Lab: Perform experiments, record observations, and data. The “FINAL LAB REPORT IS DUE IN ONE WEEK” from the experiment day. More details about the theory and postlab questions can be found in the presentation document (Module > Materials). Review the “recrystallization of benzoic acid” protocol from CHEM 3210 labs. Objectives a. Synthesize phenylmagnesium bromide, a Grignard reagent b. Syntheses of benzoic acid and recrystallization of the product (benzoic acid) Synthetic Scheme Br + Mg MgBr O + 1 CO2 HCl OH CHEM 3220 ORGANIC LABORATORY II EXPERIMENT HANDOUT UNIVERSITY OF NORTH TEXAS Reaction Theory (additional information): Part A: Synthesize phenylmagnesium bromide, a Grignard reagent Among synthetic strategies used to create new carbon-carbon bonds, Grignard reactions occupy an important place (named after the French chemist, Victor Grignard). A Grignard reaction typically requires an initial step, the in situ preparation of the Grignard reagent, RMgX. Why? These organometallic reagents are extremely reactive, and have practically no shelf-life: they must, therefore, be prepared from shelf-stable precursors, such as alkyl halides, just before the Grignard reaction is to be carried out. The process is illustrated below: δ+ R δ− X + Mg δ− R diethyl ether δ+ Mg δ− X Due to the low electronegativity of Mg, the compound is highly polar, as illustrated above: note that during its formation, the polarity at the carbon gets reversed! The unique properties of this class of compounds are due to the ability of the carbon to behave as a carbanion: Grignard reagents are thus excellent nucleophiles. This is what allows for the formation of new carboncarbon bonds when they react with compounds in which carbon has the opposite polarity. Victor Grignard, who first prepared these compounds and realized their tremendous potential in organic synthesis, was awarded the Nobel Prize in 1912. For instance, when a Grignard reagent attacks the carbonyl groups of aldehydes, ketones, esters and acyl halides, the resulting products are 1◦, 2◦, or 3◦ alcohols. When a Grignard reagent is added to carbon dioxide, the products are carboxylates that can be protonated with a strong acid to yield carboxylic acids. When choosing a solvent for a Grignard reaction, it is critical that the selected solvent be aprotic. As well as being strong nucleophiles, Grignard reagents are also extremely strong bases, capable of readily 2 CHEM 3220 ORGANIC LABORATORY II EXPERIMENT HANDOUT UNIVERSITY OF NORTH TEXAS abstracting acidic protons from protic solvents: this reaction would destroy the reagent immediately. Thus, it is imperative that all chemicals and other materials be completely dry at all stages during the experiment. Any compounds containing even minuscule amounts of acidic contaminants (e.g., water, alcohols, amines, etc.) will destroy the reagent! Consider the pKa values of the above-mentioned compounds and how easily they could be removed by a strong base, such as a Grignard reagent. RMgX + H2O R-H + MgX(OH) The ideal solvent also should be polar. A polar solvent stabilizes the Grignard reagent by coordination, which also plays a significant role during the in situ synthesis of the reagent since it is also quite soluble in the polar solvent. Non-polar solvents cannot participate in such coordination; they are also poorly able to dissolve the Grignard reagent or the highly polar, ionic products formed during Grignard reactions. Et R Et O Mg O Et X Et The most commonly used solvent for Grignard syntheses is anhydrous diethyl ether. It is both polar and aprotic, and it has the added benefit of having a low boiling point! This latter point is important because it allows the solvent to serve as an internal cooling agent: the low boiling point of the solvent keeps the reaction mixture from overheating, despite the exothermic nature of the reaction, since the temperature of the reaction mixture cannot exceed the boiling point of the solvent. The heat released during the exothermic reaction simply causes the ether to boil more vigorously. Since Grignard reagents decompose faster at elevated temperatures, it follows that solvents with low boiling points are more desirable for carrying out these reactions. In this experiment, you will synthesize benzoic acid, via a Grignard synthesis, from bromobenzene (C6H5Br) and dry ice, as illustrated in the reaction scheme below. The first step is the in situ preparation of the Grignard reagent, phenyl magnesium bromide (C6H5MgBr), from the reaction between elemental Mg and C6H5Br, using anhydrous diethyl ether as the solvent. Mg is a reactive metal, can result in the formation of a thin oxide layer. When working with powdered Mg, a stirring rod or spatula can be used to scratch the powder against the walls of the flask containing the reaction mixture, in order to expose the elemental Mg layer under the MgO film. It is visually easy to tell when the reaction starts: the solution starts to become cloudy and slowly starts to bubble. Why? The heat generated by the exothermic reaction causes the ether to soon start boiling when the reaction with Mg is almost complete, the ether will stop boiling, and you will also notice that most (but not all!) of the Mg will have disappeared. The experimental yield of the desired product is naturally diminished when side reactions occur. 3 CHEM 3220 ORGANIC LABORATORY II EXPERIMENT HANDOUT UNIVERSITY OF NORTH TEXAS In this case, it is found that a side-reaction does indeed form to an appreciable extent: the freshly formed phenyl magnesium bromide reacts with the as-yet-unreacted bromobenzene to form biphenyl, as shown below. MgBr f reshly f ormed f rom bromobenzene + Br as-yet-unreacted bromobenzene biphenyl Although it is not possible to prevent the reaction from occurring, it is possible to statistically minimize its likelihood. Instead of adding all the bromobenzene at once to the elemental magnesium, only a portion is added first, to get the reaction started; the rest is added dropwise. This method greatly decreases the amount of unreacted bromobenzene available, at any given time, to react with the newly made Grignard reagent. This technique also serves another useful purpose: it allows one to control the rate of the reaction of bromobenzene with magnesium, something which can be easily monitored visually by observing how vigorously the ether solvent is boiling. What might you do to control the reaction rate if you notice that it is boiling extremely rapidly? Materials List: Dry test tubes, bromobenzene, Mg metal or Mg powder, anhydrous ether, dry ice, glass rod, pH paper, needles (ask your TA if needles are required), hexanes, NaOH, HCl, Mel-Temp apparatus. Procedure (followed in the lab) Part 1: Preparation of the Grignard Reagent, Phenylmagnesium Bromide It is critically important that water not contaminate this part of the procedure. Before beginning this reaction, inspect the green syringe supplied in the glassware kit. If it appears wet, tell the teaching assistant and they will supply a dry one. Do not rinse or try to clean any glassware or supplies before starting this procedure. The teaching assistant will supply two glass test tubes and a glass stir rod that was heated in the oven at 110°C for at least 30 minutes before the lab. They may still be warm when you receive them. Hold them with a paper towel to cool before use. Do not rinse these tubes with water, even if they appear dirty. Sometimes the tube may be scratched and appear hazy. These tubes have been cleaned by laboratory staff and inspected by your teaching assistant. When the test tubes are room temperature, acquire magnesium from the teaching assistant. This magnesium has been dried and ground previously in a mortar and pestle. The powder or turnings should be grey with a dull metallic luster. If the magnesium appears to be a sooty black then you may need to grind them more. Ask your teaching assistant for guidance. The sooty black coating on the magnesium will slow the rate of this reaction. Grinding the metal will expose fresh surface area which reacts faster with alkyl bromides, or in this case, an aryl bromide to form Grignard reagents. Use the 4 CHEM 3220 ORGANIC LABORATORY II EXPERIMENT HANDOUT UNIVERSITY OF NORTH TEXAS mass balance and weigh paper to measure around 45-52 mg of prepared magnesium into one of the test tubes you received from your teaching assistant earlier. Record this mass for your lab report and convert this mass to mols, as it will be your limiting reagent from which theoretical yield will be calculated from. (mol of Mg = g of Mg/MM of Mg). This test tube will be referred to as test tube #1 by your teaching assistant or other places in this procedure. In your fume hood, pipette about 0.5 mL of diethyl ether into test tube #1 and cap the tube with a rubber septum. The septum should appear dry. The teaching assistant should give you a demonstration on how the tube should be capped with the septum. Make sure you understand the correct method of capping tubes with septa or you may have a spill later in this procedure. Set this tube aside in your hood for now. In your fume hood, pipette 0.75 mL of diethyl ether into the second test tube you received from the teaching assistant and cap this tube with another rubber septum. If you are not sure you have capped this test tube correctly, check with your teaching assistant. Transfer this volume from the diethyl ether bottle directly to this test tube using the graduation marks on the side to estimate the 0.75 mL. Ether may squirt from the pipette so put the bottle close to the test tube in the fume hood. Do not invert the pipette so that the diethyl ether flows into the bulb of the pipette. This test tube will sometimes be called test tube #2 by your teaching assistant or other places in this procedure. In your fume hood and using the syringe from your glassware kit with needle provided by the teaching assistant, add 0.25 mL of bromobenzene to test tube #2. You will need to carefully push the needle through the middle of the rubber septum and inject the bromobenzene into the ether waiting there. Be sure to read the volume from the base of the plunger and not the thin segment that enters into the hub or needle. Account for any air bubbles and try to avoid any if possible. It is better that you add a drop too much than too little. However, superfluous extra additions will increase the rate of formation of the biphenyl biproduct, thus lowering the yield of the target product. The bromobenzene and ether can be mixed with the needle still in the septum by gently shaking for a few seconds. Carefully in your fume hood, invert test tube #2 so that the syringe is underneath and test tube #2 is upside-down. If your septum is not correctly secured, you may spill everything. Withdraw the contents of test tube #2 into the syringe by slowly pulling back the plunger while the tip (the bevel) of the needle is below the surface of the solution. You should have about 1 mL in the syringe. Set the filled syringe on a clean paper towel in the fume hood. In the fume hood, remove the septum from test tube #1 and add approximately 0.1 mL of bromobenzene-diethyl ether solution from the syringe. Place the syringe back on the clean paper towel. Mix contents of test tube #1 with the glass rod and look for the generation of gas bubbles, but a cloudy looking solution is usually seen first. Using the dry glass stirring rod supplied by the teaching assistant, grind the magnesium at the bottom of test tube #1. It is best to place the bottom of the test tube on the paper towel and gently but firmly tap and roll the end of the stir rod into the metal. Too 5 CHEM 3220 ORGANIC LABORATORY II EXPERIMENT HANDOUT UNIVERSITY OF NORTH TEXAS much pressure may break the stir rod and if the tube is not on a hard surface, you may punch a hole in the bottom of the tube. When you begin to see gas bubbles, re-cap test tube #1 with the rubber septum and pierce it with a second needle supplied by the teaching assistant. This is not the needle attached to the syringe containing the bromobenzene-diethyl ether solution. This a syringe-less needle used to vent pressure from the ether gas being generated. Make note of any heat changes but avoid holding the syringe or test tube #1 tightly in your warm hand. The heat from your hand will cause the ether to evaporate. Once bubbles in test tube #1 are observed and the tube re-capped, pierce the septum of test tube #1 with the syringe and needle containing the bromobenzene-ether solution. You should have two needles in the septum, one with a syringe and one without. Slowly over a period of several minutes, add the remaining bromobenzene-ether solution dropwise into test tube #1. Adding the bromobenzene-diethyl ether solution too fast will increase the formation of the biphenyl biproduct. The rate of reaction should remain under control at all times. If the reaction becomes too warm and the ether is boiling away, then place test tube #1 in an ice bath until the rate slows. When all the bromobenzene-diethyl ether has been added, continue to monitor test tube #1 until the bubbling ceases and most of the magnesium metal (the limiting reagent) has been consumed in the reaction. If there is still a large amount of the original metal, inform your teaching assistant. Uncap the tube and start the reaction again by grinding if this is the case. Finally, check to see that the ether has not evaporated from test tube #1. This tube originally had 0.5 mL and 1.0 mL was added from the syringe. If test tube #1 contains less than 1 mL of volume, add more diethyl ether until there is 1.5 mL solution. The phenylmagnesium bromide now in-situ is very sensitive to water and other protonated solvents. In-situ means in this context that the Grignard reagent is “in the reaction solution” and should be used as soon as possible. Grignard reagents are normally generated in this manner because they are unstable and decompose quickly. Avoid any contact with water. Part 2: Reduction of Carbon Dioxide by the Grignard Reagent to Yield the Benzoate Salt In the fume hood, place a small piece of dry ice that has been dried thoroughly with a paper towel to remove condensation into a 30-50 mL beaker. The dry ice is very cold and should be handled carefully with a paper towel and avoid prolonged skin exposure. Remove the vent needle from test tube #1 and withdraw its contents into the syringe as done before in this procedure in part 1. Check to see that the septum is properly on the test tube before inverting it. Squirt this solution containing the in-situ Grignard reagent directly on the dry ice. Use a pipette (not the syringe and needle) to rinse test tube #1 with 1 mL of diethyl ether. Use the syringe and needle to transfer this rinse onto the dry ice also. Wait until all the dry ice sublimes before starting part 3. Prepare an ice bath in a 250 mL beaker and a hot water bath on the hot plate in a 100-150 mL beaker for Part 5 while waiting for the dry ice to sublime. Fill a test tube with deionized water and place it in the hot water bath. Add 1 mL of ethanol to a test tube and place it in the ice bath. 6 CHEM 3220 ORGANIC LABORATORY II EXPERIMENT HANDOUT UNIVERSITY OF NORTH TEXAS 7 CHEM 3220 ORGANIC LABORATORY II EXPERIMENT HANDOUT UNIVERSITY OF NORTH TEXAS Part 3: Hydrolysis of the Benzoate Salt to Yield Benzoic Acid Add 2 mL of 3 M hydrochloric acid to the 30-50 mL beaker containing the ether and benzoate salt. Stir and transfer the contents of the beaker to the glass centrifuge tube from the glassware kit. Rinse the beaker with 1 mL of diethyl ether and transfer that to the same centrifuge tube. Part 4: Removal of Magnesium salts and Biphenyl Biproduct Observe two separate layers in the centrifuge tube in part 3 and remove the aqueous layer from the bottom. If there are not two observable layers, you may need to add more ether or water or both. Check with the teaching assistant before adding additional volume to the centrifuge tube. This aqueous layer is mostly excess hydrochloric acid and salt water leaving benzoic acid, biphenyl, and unreacted bromobenzene in the ether layer. Transfer this aqueous layer to a clean 10 mL Erlenmeyer flask. This flask will be referred to as Flask #1 in the clean-up procedures. Add 1 mL of 3 M sodium hydroxide solution to the ether remaining in the centrifuge tube. Put the cap on tightly and shake gently. Open the cap occasionally to vent the gas or the cap may pop off. Using a pipette remove the aqueous layer from the bottom of the centrifuge tube and transfer it to a clean 10 mL Erlenmeyer flask (not Flask #1). This aqueous solution contains sodium benzoate (by deprotonation of benzoic acid) and the ether remaining contains the biphenyl biproduct along with unreacted bromobenzene. Wash the ether twice more with 0.5 mL of 3 M sodium hydroxide and transfer the aqueous layer on the bottom to the same flask. This flask will be referred to as Flask #2 later in the procedure. The ether now only should contain a biphenyl-bromobenzene solution and should be disposed of in the organic waste bottle. Part 5: Isolation and Recrystallization of Benzoic Acid Add 1 mL of 3 M hydrochloric acid to Flask #2 and mix to protonate the sodium benzoate. Check the pH of the solution in Flask #2 with pH paper and continue adding the 3 M hydrochloric acid solution dropwise and mix until the contents of the flask are acidic. Benzoic acid should precipitate as a solid as the benzoate is protonated. Cool Flask #2 in an ice bath for a few minutes. Use a Hirsch funnel and filter flask with the water aspirator in the fume hood to filter the contents of Flask #2 on the fritted funnel. Wash the benzoic acid with ice cold water while it is on the funnel. Allow the product to dry on the funnel for a few minutes. Save a small portion of the benzoic acid product to be used for determination of the crude products melting point. The remainder of crude product will be recrystallized from water according to the procedure learned in CHEM 3210. As a reminder, the crude benzoic acid is placed in a test tube and dissolved in the minimum amount of boiling deionized water that was prepared in a test tube at the end of Part 2 of this procedure. Use the glass stir rod to stir and mix as the boiling water is added. Leave the tube in the 8 CHEM 3220 ORGANIC LABORATORY II EXPERIMENT HANDOUT UNIVERSITY OF NORTH TEXAS hot water bath as you mix. When all the benzoic acid has dissolved, remove the tube from the hot bath. Allow it to come to room temperature undisturbed and then place it in the ice bath for two minutes. Filter the contents of the tube using the Hirsch funnel and filter flask attached to the water aspirator. Use only ice-cold water to rinse any benzoic acid from the test tube into the funnel. Let the crystalline benzoic acid dry on the funnel for two minutes, then pipette a very small amount (1-2 drops) of the ice-cold ethanol prepared at the end of Part 2 in this procedure over the crystals. If the ethanol is not cold or too much is used then your product will dissolve and be lost. Let the crystals dry for two more minutes on the funnel. If moisture remains on the product, use a filter paper provided by the teaching assistant to blot the crystals dry. Use the mass balance to measure the mass of your final benzoic acid. Record this in your lab report and use this information along with the mass and mols of magnesium used in Part 1 of this procedure to calculate percent yield. This must be included in your lab report. Determine the melting point of both the crude and recrystallized benzoic acid using the MelTemp apparatus. Follow the same procedures taught in 3210. Ask your teaching assistant for help, if needed. Part 6: Cleaning Up Dispose of any unused organic material in the appropriate waste container. Test tube #1, #2, and the glass stir rod should be rinsed with acetone and ethanol. Dispose of the rinse solvents in the organic chemical waste bottle, then allow the glassware to dry on a paper towel. Return the test tubes and stir rod to your TA so they can be prepared in the oven for the next class. Do not put anything in the oven yourself. Flask #1 and the liquid in the filter flask can be rinsed with a copious amount of water into the sink. Rinse the filter flask and the Hirsch funnel with acetone and ethanol with the waste going into the organic chemical waste bottle. Flask #2 should be washed like test tube #1 and #2, but return both flasks to the glassware kits. If the glass still did not appear clean, let the teaching assistant know so the laboratory supervisor can attempt to clean it with harsher methods. All needles should be returned to the safety caps and disposed of in the needle waste container. Do not wash the syringe with water. It should be empty and returned to the glassware kit. Used capillary tubes and used pipettes are disposed of in the broken glass waste box. Check to make sure all caps and lids are on chemical bottles in the fume hood. Wipe down the areas you worked with a damp (not wet) paper towel and dispose of the towel in the regular trash can unless you cleaned a big spill. In that case, you need to inform the teaching assistant. Disassemble any clamps that were used in the fume hood. The thermometer should be stored upright in the Mel-Temp apparatus. If it was used for something else, make sure it is clean and put it back in the Mel-Temp. 9 CHEM 3220 ORGANIC LABORATORY II EXPERIMENT HANDOUT UNIVERSITY OF NORTH TEXAS Turn the Mel-temp apparatus to a power level of ZERO and turn it off. Unplug everything from the fume hood and turn off the light. Check with your teaching assistant before leaving. Throw used gloves in the trash can and wash your hands. Additional Information and Postlab Questions Postlab Questions: 1. List the major by-products expected during this reaction and how to minimize their formation. What measurements or data can help understand formation of byproduct(s). (Note: full points for explaining the formation of by-products Benzoic acid mp – 122°C (252°F) HAZARDS/SAFETY: Diethyl Ether: Highly flammable; Hexane: Flammable; Bromobenzene: Irritant. Dry ice can burn your skin, don’t touch with the bare hands. NaOH and HCl- Strong dehydrating agent - Corrosive to tissues, do not touch any reagents/chemicals directly - All safety rules listed in the safety Rules agreement will be strictly enforced. Start your experiments only after TA’s approval Talk to your TA if you have any health concerns. Please avoid crowding at wash stations and waste disposal hood or other common areas. Wait for your turn for using the balance or any other common item/materials in the lab. Ask your TA if you have any questions regarding the procedure before starting the experiment. Handle the dry test tubes carefully. Follow your TA instructions strictly for the handling of the apparatus/chemicals/ disposal of the chemicals. Do not remove your safety goggles during any time in the lab. Wash your hands thoroughly with soap before stepping out of the labs . Do not taste or smell the samples. Please follow TA’s instructions for careful disposal of reactive RMgX compound. Be careful while working with sharp syringes, Dispose of needles into designated boxes. Wash hands before leaving the lab Additional Feedback Note: Do not use an excess of Mg turnings or excess of Mg powder TA’s should show the student how to cap the TT’s, TA’s should show the student how to use a syringe to release the pressure. If required, remind students on setting up the vacuum filtration If required, remind students of the technical details of measuring the melting point (filling the capillary tubes and setting the knob) 10 CHEM 3220 ORGANIC LABORATORY II EXPERIMENT HANDOUT UNIVERSITY OF NORTH TEXAS Make sure the product is fully dry, calculate the yield – this will help for avoiding over 100% yield and even helps with the mp analysis – Follow your TA’s advice. 11