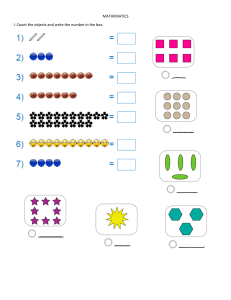
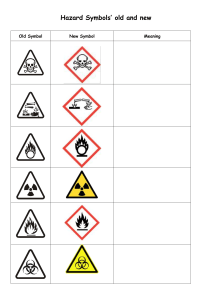
List of Common Polyatomic Ions: Cations: Symbol NH4+ Name Ammonium Charge +1 Symbol Hg2+2 Name Charge Dimercury (I) +2 Anions: Symbol C2H3O2C6H5COOHCO3HSO4HSO3BrO3ClO3ClO2CNH2PO4OHClOIO3NO3NO2ClO4IO4MnO4SCNFe(CN)63Fe(CN)64- Name Charge Acetate -1 Benzoate -1 Bicarbonate -1 Bisulfate -1 Bisulfite -1 Bromate -1 Chlorate -1 Chlorite -1 Cyanide -1 Dihydrogen Phosphate -1 Hydroxide -1 Hypochlorite -1 Iodate -1 Nitrate -1 Nitrite -1 Perchlorate -1 Periodate -1 Permanganate -1 Thiocyanate -1 Hexacyanoferrate (III) Hexacyanoferrate (II) Variable Valence Cations: Antimony (III) and (V) Arsenic (III) and (V) Cobalt (II) and (III) Chromium (II), (III), and (VI) Copper (I) and (II) Gold (I) and (III) Indium (I) and (III) Iron (II) and (III) Lead (II) and (IV) Manganese (II), (III), (IV), and (VIII) Mercury (II) Hg2+ Mercury (I) Hg22+ Platinum (II) and (IV) Tin (II) and (IV) ✤Silver (1+) ✤Zinc (2+) ✤Cadmium (2+) ✤Forms only one type of ion and Roman numerals are not used. Symbol CO32CrO42Cr2O72HPO42MoO42C2O42O22SiO32SO42SO32S2O32WO42AsO43AsO33BO33PO43PO33- Name Charge Carbonate -2 Chromate -2 Dichromate -2 Hydrogen Phosphate -2 Molybdate -2 Oxalate -2 Peroxide -2 Silicate -2 Sulfate -2 Sulfite -2 Thiosulfate -2 Tungstate -2 Arsenate -3 Arsenite -3 Borate -3 Phosphate -3 Phosphite -3 Diatomic Molecules: -3 -4 Bromine Chlorine Fluorine Hydrogen Iodine Nitrogen Oxygen Br2 Cl2 F2 H2 I2 N2 O2 Prefixes (for non-metals): 1 – Mono 2 – Di 3 – Tri 4 – Tetra 5 – Penta 6 – Hexa 7 – Hepta 8 – Octa 9 – Nona 10 – Deca Roman Numerals: I One IV Four II Two V Five III Three VI Six VII Seven VIII Eight IX Nine X Ten