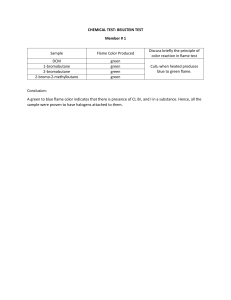
Name: ______________________________________ Date: _______________ Flame Test Lab Chemists began studying colored flames in the 18th century and soon used "flame tests" to distinguish between some elements. Different elements burn with different colored flames. Although some of the flames you will be seeing will appear similar in color, their light can be resolved (separated) with a prism into distinctly different bands of colors on the electromagnetic spectrum (ROYGBIV). These bands of colors are called atomic line spectra, and they are UNIQUE to each element. Violet indigo blue green yellow orange red Niels Bohr studied the line spectrum for hydrogen, and wondered what the specific line spectrum had to do with the structure of the atom. He postulated that an electron can have only specific energy values in an atom, which are called energy levels. Bohr believed that the energy levels for electrons were quantized, meaning that only certain, specific energy levels were possible. Also, the further away from the nucleus, the more energy the electrons have. How does an electron move between energy levels? By gaining the right amount of energy, an electron can move, or undergo a transition, from one energy level to the next. We can explain the emission of the light by atoms to give the line spectrum like this: 1. An electron in a high energy level (excited state) undergoes a transition to a low energy level (ground state). The transition is instantaneous & complete. 2. In this process, the electron loses energy, which is emitted as a photon of light (a particle which behaves like a wave) 3. The energy difference between the high energy level and the low energy level is related to the frequency (color) of the emitted light. Pre-Lab Questions: 1. Bohr's important discovery was that energy levels of electrons are quantized (only existing in certain, specific levels). In what year was this discovery made? 2. What happens to an electron when energy is added? 3. What is released when an electron loses energy? 4. What determines the frequency (color) of photons? The Flame Test Objective: To identify atoms in solutions using the colors they emit when heated. Safety: 1. Follow all lab safety rules. 2. Place only the wire loop in the flame. 3. Wear apron and safety goggles at all times. 4. Do not knock over the flame, reach over it, or clutter your lab area with paper Instructions for flame tests: 1. You are going to find out what color flame six different known samples make. Then, you will use your results to determine which metal is in the “unknown” sample. 2. Clean the metal loop in the hottest part of the flame. Be sure not to cross-contaminate the samples. 3. Dip the wire loop in the solution and hold it over the flame. Observe and record the color. 4. Once you have collected your data for the “known” samples, test your unknown solution. Record the unknown sample number, color and element in the data table. Solution Strontium Calcium Lithium Sodium Copper Potassium Unknown #____ Color of Flame Which atom is it? Sr2+ Ca2+ Li+ Na+ Cu2+ K+ Post Lab Questions: 1. Why do atoms give off light energy when heated? (Use “electrons” somewhere in your answer.) 2. Explain what could happen if the wire is not properly cleaned? 3. Explain why different atoms give off different colors of light? 4. Write the isotopic notation, Lewis dot structure, and the electron configuration for all the elements tested (except copper) on the back of this page. 5. Is the change observed in this lab a physical change or a chemical change? Explain your answer. 6. Why do you think that we used the metal salts instead of the pure metals? (Think about the properties of the pure elements) What do the street lamp, salt, and fireworks have in common? They all contain SODIUM, which gives off a unique ORANGE flame when heated. You are going to find out what color FLAME six different known samples make. You will then use your results to work out which metal is in the unknown sample. Your first Job is to make sure that your flame test wire is CLEAN. Do this by holding the metal loop in the hottest part of the Bunsen burner flame. If it is clean there should be no change in the color of the flame when the metal loop is put into the flame. If the metal loop is NOT CLEAN, clean it by dipping it into the HCl solution provided then holding it in the burner flame until there is no change in the color of the flame. You should have different loops for each sample. DO NOT cross contaminate samples by using different loops then the ones assigned to the sample. Your next job is to do the flame tests. Dip the wire loop into one of the known solutions. Then hold the metal loop in the hottest part of the Bunsen burner flame. Make a note of the color in the FLAME TEST CHART. Continue to test the other known solutions. Keep going until you have recorded the color of al of the known solutions. Repeat job 2 but use the unknown solution. Can you figure out what metal is in this solution(don’t forget to record the sample # in your chart) Post-Lab Question #4: Element Example: Nitrogen Isotopic Notation Calcium Lithium Sodium Potassium Electron Configuration 14 7 Strontium Lewis Dot Structure N 1s22s22p3 1.