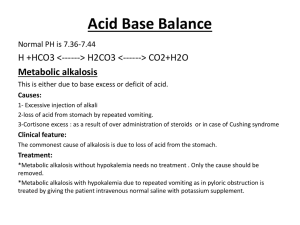
iClicker / Jeopardy Questions 1. Kidneys filter how much plasma in the normal adult? = 180L 2. An EKG change of tall tented T wave is indicative of? = hyperkalemia 3. A pt with COPD is susceptible to developing CO2 retention which in turn causes this? - Respiratory acidosis 4. This is the best indicator for fluid balance - weight 5. Sudden weight gain, tachycardia, distended jugular vein, edema, and crackles are signs of this hypervolemia 6. This electrolyte helps with metabolism of carbs and protein as well as helps with cardiac function and muscle relaxation -- magnesium 7. Magnesium - 1.5 - 2.5 meq/ dL 8. TEXTBOOK NOTES ★ Factors that influence amt of body fluid = Age, gender, body fat ○ Older age, female gender, higher fat cells --> less body fluid ○ Younger age, male gender, less fat cells --> higher body fluid ★ Body fluid is located in ICF & ECF. Most of it is in ICF. ★ ECF has 3 compartments: intravascular, interstitial & transcellular fluid space ■ Intravascular = plasma + blood ■ Interstitial = lymph ■ Transcellular = CSF, pericardial, synovial etc ○ ECF transports electrolytes, hormones & enzymes ★ Body fluid normally moves b/w those 2 compartments (ECF & ICF) to main equilibrium b/w spaces ○ Loss of fluid can disrupt equilibrium ○ Loss of ECF into a space that doesn’t help with this equilibrium is called third-space fluid shift or third spacing ■ Evidence in body of 3rd spacing --> decrease in urine output despite increase in fluid intake ○ Major electrolytes in ECF = Na+ ions ■ outnumbers the other ions in the ECF, so it affects the overall concentration of ECF. ■ Na+ retention is associated w/fluid retention. Loss of Na+ is associated w/ decreased volume of body fluid. ■ ECF has low #s of K+ so it can only tolerate small changes in concentration. So the release of large stores of intracellular K+ caused by trauma to cells & tissues can be extremely dangerous. ○ Major electrolytes in ICF = K+ & PO4 ★ The human body maintains Na+ & K+ equilibrium through sodium-potassium pumps. 1. Differentiate between osmosis, diffusion, filtration, and active transport. ★ OSMOSIS= water going down the concentration gradient ○ Magnitude of osmosis depends on # of particles and not their weight ○ Osmolality = # of dissolved particles in a unit of fluid ○ Tonicity = measure of the solutes’ osmotic pressure ■ It’s control determines normal state of cellular hydration & cell size ○ Na+, mannitol, glucose, sorbitol are effective osmoles (capable of affecting water movement) ○ Osmotic pressure = is the amount of hydrostatic pressure needed to stop the flow of water by osmosis. It is primarily determined by the concentration of solutes. ○ Oncotic pressure is the osmotic pressure exerted by proteins (e.g., albumin). ○ Osmotic diuresis is the increase in urine output caused by the excretion of substances, such as glucose, mannitol, or contrast agents in the urine. DIFFUSION: - Occurs when substances go down their concentration gradient Occurs through random movement of ions and molecules FILTRATION: - Hydrostatic pressure in capillaries filters fluid out of the intravascular compartment (plasma + blood) & into the interstitial fluid (lymph). ACTIVE TRANSPORT: - energy must be expended for the movement to occur against a concentration gradient. Cuz NA+ concentration is greater in ECF than ICF, it tends to go down its gradient & enter the cells. This is offset by the Na+K+ pumps which put Na+ back from the cells to the ECF. The same things applies to K+ 2. Describe the role of the kidneys, lungs, and endocrine glands in regulating the body’s fluid composition and volume. Kidney: ● ● One of the organs responsible for homeostasis of the composition and volume of body fluids → keeping them within their narrow limits of norm FXN: Maintain normal fluid balance, including: ○ Regulation of ECF volume and osmolality (solute concentration per kg in blood and urine) by selective retention and excretion of body fluids ○ Regulation of normal electrolyte levels in the ECF by selective electrolyte retention and excretion ○ Regulation of pH of the ECF by retention of hydrogen ions ○ Excretion of metabolic wastes and toxic substances ■ ■ ● ● Kidneys act anonymously and in response to hormones (bloodborne messengers, ex aldosterone and antidiuretic hormone (ADH) ● EX: Renin-Angiotensin-Aldosterone System: ○ Juxtaglomerular cells of the kidneys release renin in response to decreased renal perfusion. ■ Renin converts Angiotensinogen (from the liver_ → Angiotensin I ■ Angiotensin-converting enzyme (ACE) converts angiotensin I → angiotensin II ● Angiotensin II with its vasoconstrictor properties, increases arterial perfusion pressure and stimulates thirst ■ As the sympathetic nervous system is stimulated, aldosterone is released in response to an increased release of renin. ● Aldosterone is a volume regulator and is also released as serum potassium increases, serum sodium decreases, or adrenocorticotropic hormone (ACTH) increases. Therefore, kidney failure leads to multiple fluid and electrolyte abnormalities Filter 180L of plasma per 24 hours. (every day) Excrete 1 to 2 L of urine daily in adults. ○ General RULE: the output is approximately 1 mL of urine per kg of body weight, per hour in all age groups Lungs: ● ● Lungs normally eliminate water vapor (insensible loss) at a rate of approx. 300 mL every day ○ More water is lost when resp. Rate increases or depth increases, or in dry climate. ■ Ex: hyperpnea→ abnormal deep respiration &/or continuous cough, increases water loss via lungs ■ Ex 2: Mechanical Ventilation, with excessive moisture decreases it. Lungs also work to maintain acid-base balance ○ Control CO2 levels in the body. ■ CO2 is an acid. ● When enters the body it combines with H2O which forms H2CO3 or carbonic acid ○ ↑ CO2 → ↑ more carbonic acid in the blood→ ↓ pH of blood (acidotic) ■ Hypoventilation: ● Not breathing enough, and too much CO2 is accumulating ○ ↓ CO2 → ↓ more carbonic acid in the blood→ ↑ HCO3- in blood (bicarbonate base)--> ↑ pH of blood (alkalotic) ■ Hyperventilation ● Breathing too much, blowing off too much CO2 Endocrine: 1. Pituitary: a. Hypothalamus makes ADH, but stores in posterior pituitary i. ADH (Antiduiretic hormone): FXN: 1. Maintains the osmotic pressure of the cells by controlling the retention or excretion of water by the kidneys and by regulating blood volume a. ADH is the most significant factor in determining whether the urine that is excreted is concentrated or dilute. b. How is ADH released? i. The osmoreceptors on the surface of hypothalamus sense changes in sodium concentration . 1. Osmotic pressure ↑, neurons become dehydrated and tell post. Pituitary to increase release of ADH. 2. ADH then goes to kidneys and carries out its fxn 2. Adrenals: a. Secrete Aldosterone from the zona glomerulosa of adrenal cortex i. Effects fluid balance by: causes sodium retention (and thus H20 retention) and Potassium loss. 1. Decrease in aldosterone causes reverse effect. ii. Released when: 1. Renin is released from kidneys due to ↓ blood flow to kidneys, or 2. serum potassium increases, or 3. serum sodium decreases, or 4. adrenocorticotropic hormone (ACTH) increases. b. Secrete Cortisol: i. Has less mineralocorticoid action, when secreted in large amounts (or given as corticosteroid therapy), can produce Sodium (Na+) and fluid retention 3. Parathyroid: a. Secrete Parathyroid hormone (PTH): i. Regulates calcium and phosphate balance by bone reabsorption, calcium absorption from the intestines, and calcium reabsorption from the renal tubules ii. 4. Hypothalamus: a. Thirst center is located here, therefore the hypothalamus controls oral intake. i. Neurons in Hypothalamus are Stimulated by intracellular dehydration when serum concentration or osmolality increases, or blood volume decreases. 1. This causes person to feel thirsty and increase their intake of oral fluids 3. Identify the effects of aging on fluid and electrolyte regulation. ★ Physiological changes of aging such as: ○ Decreased respiration function --> impaired pH regulation in older adults with major trauma/illness ○ Decline in renal fxn, muscle mass & daily exogenous creatinine production. ■ So high-normal & minimally elevated serum creatinine values can indicate substantially reduced renal fxn in older adults. ○ Decrease in age-related muscle mass--> lower concentration of body fluid ○ Use of multiple medications can affect renal & cardiac fxn --> increased risk for fluid-electrolyte disturbances ○ Dehydration is common ■ Due to decrease in kidney mass, GFR, renal blood flow, decreased ability to concentrate urine, inability to conserve Na+, decreased excretion of K+, and of decrease in total-body water. ○ Loss of subcutaneous supporting tissue and resultant thinning of the skin occurs; the dermis is dehydrated and loses strength and elasticity. ★ Clinical manifestations of fluid-electrolyte imbalances may be different in older adults vs young or middle aged populations ○ Routine procedures like laxatives/enema before colon-X ray can produce a serious FVD ○ The signs can be subtle or atypical. ○ Rapid infusion of excessive volume of IV fluids can lead to fluid overload & cardiac failure in older pts. ■ This can happen with smaller volumes of fluids & more quickly in older adults compared to other age groups because of the decreased cardiac reserve & reduced renal fxn. 4. Plan effective care of patients with the following imbalances: fluid volume deficit and fluid volume excess, sodium deficit (hyponatremia) and sodium excess (hypernatremia), and potassium deficit (hypokalemia) and potassium excess (hyperkalemia). TABLE 13-4 Fluid Volume Disturbances Imbalance Contributing Factors Signs/Symptoms and Laboratory Findings Fluid volume Loss of water and electrolytes: as in Acute weight loss, ↓ skin turgor, deficit vomiting, diarrhea, fistulas, fever, excess oliguria, (hypovolemia sweating, loss, capillary filling time prolonged, gastrointestinal suction, and third-space low CVP, ↓ BP, flattened neck fluid shifts; and decreased intake, as in veins, dizziness, weakness, thirst anorexia, nausea, and inability to gain and confusion, ↑ pulse, muscle fluid. Diabetes insipidus cramps, sunken eyes, nausea, access to (cannot burns, concentrate blood urine) and uncontrolled diabetes both contribute to a depletion of extracellular fluid volume. increased concentrated urine, temperature; cool, clammy, pale skin Labs indicate: ↑ hemoglobin and hematocrit, ↑ serum and urine osmolality and specific gravity, ↓ urine sodium, ↑ BUN and creatinine, ↑ urine specific gravity and osmolality Fluid volume Compromised regulatory mechanisms, such as Acute weight gain, peripheral edema excess kidney injury, heart failure, and cirrhosis; and (hypervolemia overzealous veins, crackles, elevated CVP, administration of ascites, breath, jugular sodium-containing fluids; and fluid shifts shortness (i.e., treatment of burns). Prolonged bounding pulse and cough, ↑ corticosteroid therapy, severe stress, and respiratory rate, ↑ urine output hyperaldosteronism augment fluid volume Labs indicate: ↓ hemoglobin and excess. of distended ↑ BP, hematocrit, ↓ serum and urine osmolality, ↓ urine sodium and specific gravity Fluid Volume Deficit (FVD) or Hypovolemia: -FVD occurs when loss of ECF volume exceeds the intake of fluid. ○ Both water and electrolytes are lost in the same proportion as they exist in normal body fluids, therefore ,the ratio of serum electrolytes to water remains the same. ■ THIS IS NOT THE SAME AS DEHYDRATION= water loss alone, with increased Na+ levels -Fluid loss of greater than 25% of the intravascular volume or when fluid loss is rapid ● ● ● ● Clinical Manifestations: can develop rapidly and severity depends on degree of lost fluid Assessment and Diagnostic Findings: ○ Labs: look at BUN and its relation to creatinine level concentration ■ Normal BUN to serum creatinine concentration ratio is 20:1. ● Patient with FVD has a BUN elevated out of proportion to serum creatinine, a ratio greater than 20:1. ■ Hematocrit level is greater than normal because there is a decreased plasma volume, in a FVD patients ■ Potassium and sodium levels can be reduced or elevated ● Hypokalemia occurs when GI and renal losses ● Hyperkalemia occurs with adrenal insufficiency ● Hyponatremia occurs with increased thirst and ADH release ● Hypernatremia results from increased insensible losses and diabetes insipidus ■ Urine output could be decreased (oliguria) ■ Specific gravity of urine is increased, and osmolarity is greater than 450 mOsm/kg because of the kidneys attempt to conserve water due to the FVD. ■ Medical Management: ○ All factors are considered including the FVD ■ If not severe, oral replacement of fluids is preferred (if patient has swallow ability) ● Fluid type depends on what fluid/electrolytes the patient is missing ● If patient is reluctant due to oral discomfort, then nurse will assist with frequent mouth care and provide nonirritating fluids. ○ Ex: fluids are Rehydrate, Elete, and Cytomax ■ For acute and chronic FVD, IV route is required ● Treatment FLUIDS are: Isotonic electrolyte solutions (e.g., lactated Ringer solution, 0.9% sodium chloride) are frequently the first-line choice to treat the hypotensive patient with FVD because they expand plasma volume ○ As soon as the patient becomes normotensive, a hypotonic electrolyte solution (e.g., 0.45% sodium chloride) is often used to provide both electrolytes and water for renal excretion of metabolic wastes. ○ Further Assessments needed during the fluid replacement TX mentioned above : ■ I&O, weight, vital signs, Central Venous pressure, LOC, breath sounds, and skin color Nursing Management: ○ To assess for FVD, nurse monitor and measure I&O q 8 hrs and/or even daily ■ As FVD develops, body fluid losses exceed fluid intake through excessive urination (polyuria), diarrhea, vomiting, or other mechanisms. Once FVD has developed, the kidneys attempt to conserve body fluids, leading to a urine output of less than 1 mL/kg/h in an adult. Urine in this instance is concentrated and represents a healthy renal response ○ ● Assess body weights daily ■ an acute loss of 0.5 kg (1.1 lb) represents a fluid loss of approximately 500 mL (1 L of fluid weighs approximately 1 kg, or 2.2 lb). ○ Vital signs are closely monitored: ■ FVD causes weak, rapid pulses and orthostatic hypotension ■ Temperature could possibly decrease as well ○ Skin and tongue turgor monitored ■ turgor is best measured by pinching the skin over the sternum, inner aspects of the thighs, or forehead ■ Tongue turgor: a normal tongue has one longitudinal furrow, and patient with FVD has many furrows ○ Urine concentration is monitored: ■ FVD patients have urine with specific gravity greater than 1.020 ○ Mental function is eventually affected, resulting in delirium in severe FVD as a result of decreasing cerebral perfusion, causes: ■ Cold extremities ■ Low central venous pressure Prevention: ■ Minimize fluid loss, Diarrhea etc. Hypervolemia (FVE) aka Fluid Volume excess : - ● ● ● FVE: refers to an isotonic expansion of the ECF caused by the abnormal retention of water and sodium in approximately the same proportions in which they normally exist in the ECF. - Usually secondary to increase Na+ retention Patho: ○ Due to simple fluid overload or diminished fxn of the homeostatic mechanisms responsible for regulating fluid balance. ■ Contributing factors: ● Heart failure ● Kidney injury ● Cirrhosis of liver ● Consumption of large amounts of salts ● Excessive fluid admin in a patient with impaired homeostasis mechanisms. Clinical Manifestations: ○ Edema ○ Distended neck veins ○ Crackles (abnormal lung sounds) Medical Management: ○ Directed at the cause. ○ Symptomatic treatment includes admin of diuretics and restricting fluids and sodium ○ A) Pharmacologic therapy: ■ Diuretics: ● Usually when sodium restriction is insufficient to reduce edema ● Diuretic choice depends on: severity of FVE, degree of impairment of renal function, and the potency of the diuretic. ■ ● ● Azotemia (increased nitrogen levels in the blood) can occur with FVE when urea and creatinine are not excreted owing to decreased perfusion by the kidneys and decreased excretion of wastes. ■ High uric acid levels (hyperuricemia) can also occur from increased reabsorption and decreased excretion of uric acid by the kidneys. ○ B) Dialysis: ■ renal function is so severely impaired that pharmacologic agents cannot act efficiently, hemodialysis or peritoneal dialysis, is used ○ C) Nutritional therapy: ■ Na+ restriction ■ Nursing Management: PLAN OF CARE ○ PLAN OF CARE: promoting rest, restricting sodium intake, monitoring parenteral fluid therapy, and administering appropriate medications ■ Rest favors diuresis of fluid→ diminished venous pooling and the subsequent increase in effective circulating blood volume and renal perfusion ■ Monitor medications diuretics: ○ Nurse would : ■ measures I&O at regular intervals ■ patient is weighed daily, ● acute weight gain of 1 kg (2.2 lb) is equivalent to a gain of approximately 1 L of fluid. ■ Breath sounds assessed at reg intervals ■ Degree of Edema, ● feet and ankles in ambulatory patients and the sacral region in patients confined to bed ■ If dyspnea or orthopnea is present, the patient is placed in a semi-Fowler position to promote lung expansion ■ Educate patient to recognize symptoms: ● Edema Prevention ○ Adherence to diet ○ avoid over-the-counter (OTC) medications without first checking with a health care provider, because they may contain sodium (e.g., Alka-Seltzer) Sodium -Na+ is most abundant in ECF→ RANGE 135-145 mEq/L -Primary determinant ECF volume and osmolality -Sodium is regulated by ADH, thirst, and the renin–angiotensin–aldosterone system. -water always follows sodium -FXNs at establishing the electrochemical state necessary for muscle contraction and the transmission of nerve impulses Sodium deficit (hyponatremia) -Serum < 135 mEq/L -Acute and chronic forms: -Acute: fluid overload in surg patient -Chronic: more frequently in patients outside the hospital setting, has a longer duration, and has less serious neurological sequelae -Also exercised-associated hyponatremia, which is more frequently found in women and those of smaller stature. But also extreme temps, with excessive water and hard exercise ● ● Patho: ○ Occurs primarily due to imbalance of water. ○ Possible cause: ■ 1. Rental loss: ● Use urine to tell if renal problems or nonrenal. ○ Low urine sodium = kidneys are retaining sodium to compensate for non-renal fluid loss (diarrhea, vomiting, sweating) ○ High urine sodium conc. = renal salt waisting ■ 2. Aldosterone deficiency ■ 3. Certain meds → anticonvulsants, oxcarbazepine, SSRI’s, etc. ■ 4. Syndrome of inappropriate secretion of antidiuretic hormone (SIADH): ● In hyponatremia & hypernatremia ○ When there is a decrease in the circulating plasma osmolality, blood volume, or blood pressure, arginine vasopressin (AVP) is released from the posterior pituitary. Oversecretion of AVP can cause excessive ADH activity, with water retention and dilutional hyponatremia, and inappropriate urinary excretion of sodium in the presence of hyponatremia. ○ SIADH can result from: ■ either sustained secretion of ADH by the hypothalamus OR ■ Production of an ADH-like substance from a tumor Clinical Manifestation: ○ depend on the cause, magnitude, and speed with which the deficit occurs ■ Poor skin turgor ■ Dry mucosa ■ Headache ■ Decreased saliva production ■ Orthostatic fall in BP ■ Neuro: siezures and coma ■ Heart: tachycardia and weak pulses ■ ■ ■ ■ ■ ■ ● ● Respiratory arrest Fatigue Nausea Vomiting Abdominal cramps Neurologic changes: ● altered mental status, status epilepticus, and coma, are probably related to the cellular swelling and cerebral edema associated with hyponatremia ○ Acute decreases in sodium, developing in less than 48 hours, may be associated with brain herniation and compression of midbrain structures. ○ Chronic decreases in sodium, developing over 48 hours or more, can occur in status epilepticus and other neurologic conditions. ■ When less than 115 mEq/L (115 mmol/L), signs of increasing intracranial pressure: such as lethargy, confusion, muscle twitching, focal weakness, hemiparesis, papilledema, seizures, and death, may occur. Assessment Findings: ○ Serum Na+ <135 ○ Serum osmolality is also decreased, except in azotemia with the accumulation of toxins. ○ if hyponatremia is due primarily to sodium loss→ urinary sodium content is less than 20 mEq/L (20 mmol/L), suggesting increased proximal reabsorption of sodium secondary to ECF volume depletion, and the specific gravity is low (1.002 to 1.004) ○ If due to SIADH: urinary sodium content is greater than 20 mEq/L, and the urine specific gravity is usually greater than 1.012 Medical Management: ○ 1. Sodium Replacement: ■ Admin Na+ by mouth, NG tube, or Parenteral route. ● Those who can eat, eat it ● Can't eat→ lactated Ringer solution or isotonic saline (0.9% sodium chloride) solution may be prescribed ○ Sodium levels must not be increased by more than 12 mEq/L in 24 hours to avoid neurologic damage due to demyelination ■ This can produce lesions that show symmetric myelin destruction affecting all fiber tracts that can present with altered cognition and decreased alertness, ataxia, paraparesis, dysarthria, horizontal gaze paralysis, pseudobulbar palsy, and coma ■ With SIADH: serum replacement isn't enough, lithium (Lithobid) needs to be admin too bec. it can antagonize the osmotic effect of ADH on the medullary collecting tubule. ○ 2. Water restriction: ■ In pat with norm FV, hyponatremia treated by restricting fluid ■ if neurologic symptoms are severe (e.g., seizures, delirium, coma), or in patients with traumatic brain injury, it may be necessary to administer small volumes of a hypertonic sodium solution with the goal of alleviating cerebral edema ○ ● 3. Pharm therapy: ■ AVP receptor antagonists are pharmacologic agents that treat hyponatremia by stimulating free water excretion Nursing Management: ○ Monitor I&O ○ Monitor daily weights ○ Monitory labs, ○ Be alert for GI manifestations: anorexia, nausea, vomiting, abdomen cramping ○ Be alert CNS changes: lethargy, confusion, muscle twitching, seizures. ■ Common in fast decreasing Na+ levels, due to Fluid overload. ○ Urine sodium and Specific Gravity may be monitored too ○ If on lithium, nurse observes for lithium toxicity, when sodium is lost ■ Supp salt given ○ Get a thorough history if they are a performance athlete→ use salt tabs ○ Geriatrics: ■ Higher risk due to decreased renal fxn and inability to excrete excess fluids ■ Hyponatremia causes confusion, ■ OTC med can cause Na+ loss too ● PLus they have a diminished sense of thirst Sodium excess (hypernatremia) ● ● Sodium > 145 mEw/L Caused by: gain of sodium in excess of water or by a loss of water in excess of sodium - Occurs in pat with FVE and FVD Patho : ○ Common cause is fluid deprivation in patients who cannot respond to thirst ■ Most older age, or young or cognitively impaired ○ Other causes: ■ Administration of hypertonic enteral feedings without adequate water ■ Loss of fluid: diarrhea ■ diabetes insipidus, and/or ADH insufficiency ■ Renal failure ■ Too much high sodium foods ■ Aldosterone excess ■ heat stroke, near drowning in seawater, and malfunction of hemodialysis or peritoneal dialysis systems ■ IV administration of hypertonic saline or excessive use of sodium bicarbonate ■ Exertional dysnatremia Clinical Manifestations: BIG AND SWOLLEN Patients ○ Hypernatremia causes increased plasma osmolality by an increase in plasma sodium conc. ■ Causes cellular dehydration and conc. of ECF, ● Dehydration commonly overlooked as the cause of mental status and behavioral changes in older patients ■ Patient is very thirsty too when sodium level is elevated (POLYDIPSIA ● Thirst mechanism decreases with age why older patients get hypernatremia ● ● ■ Elevated body temp ■ Swollen dry tongue and sticky mucous mem ■ Edema ■ Hallucinations ■ Lethargy ■ Nausea ■ Vomiting ■ Increased muscle tone ○ Diagnostic findings: ■ Sodium level ↑, serum osmolality ↑, and urine SG and osmolality increase as kidneys try to keep water. Medical Management: ○ RX: gradually lower serum sodium level by the infusion of a hypotonic electrolyte solution (e.g., 0.3% sodium chloride) or an isotonic non saline solution (e.g., dextrose 5% in water [D5W]). ■ D5W used when water needs to be replaced without sodium ■ Hypotonic sodium solution is gradually reducing the serum sodium level, thereby decreasing the risk of cerebral edema Nursing Management: ○ I& O monitored ○ Watch OTC meds ○ Obtain med hx because some prescription medications have a high sodium content ○ Note the patient’s thirst or elevated body temperature ○ monitors the patient closely for changes in behavior, such as restlessness, disorientation, and lethargy. ● Prevention ○ Nurses prevent by providing oral fluids at regular intervals ○ For patients with diabetes insipidus, adequate water intake must be ensured Potassium Major intracellular electrolyte, only 2% in ECF - Concentration ranges from 3.5-5 mEq/L - Even minor variations are SIGNIFICANT Important in neuromuscular function - Influences both skeletal and cardiac muscle activity Imbalances commonly associated with diseases, injuries, meds (NSAIDS and ACE inhib) and acid-base imbalances - 80% of K+ is excreted daily leaves through kidneys, 20% excreted through bowel and in sweat - Because the kidneys do not conserve potassium as well as they conserve sodium, potassium may still be lost in urine in the presence of a potassium deficit. TABLE 13-7 Potassium Imbalances Imbalance Contributing Factors Potassium Diarrhea, vomiting, Signs/Symptoms gastric suction, Fatigue, anorexia, nausea deficit corticosteroid (hypokalem hyperaldosteronism, ia) amphotericin B, bulimia, osmotic motility, ventricular asystole or diuresis, fibrillation, Serum potassium administration, vomiting, muscle carbenicillin, polyuria, decreased and alkalosis, starvation, diuretics, and digoxin toxicity weakness, bowel paresthesias, leg cramps, ↓ BP, ileus, abdominal <3.5 distention, hypoactive reflexes. mEq/L ECG: flattened prominent T U waves, waves, ST depression, prolonged PR interval Potassium Pseudohyperkalemia, oliguric kidney Muscle weakness, tachycardia → excess injury, use of potassium-conserving bradycardia, dysrhythmias, flaccid (hyperkalem diuretics paralysis, paresthesias, intestinal ia) insufficiency, Serum in patients with metabolic renal acidosis, Addison disease, crush injury, burns, colic, cramps, abdominal distention, irritability, anxiety. potassium stored bank blood transfusions, rapid ECG: tall tented T waves, prolonged >5.0 mEq/L IV administration of potassium, and PR interval and QRS duration, certain medications such as ACE absent P waves, ST depression inhibitors, NSAIDs, cyclosporine Potassium deficit (hypokalemia) LOW AND SLOW Patients - Hypokalemia, usually indicates a deficit in total potassium stores. CAN OCCUR in patients with normal potassium stores: When alkalosis (high blood pH) is present, a temporary shift of serum potassium into the cells occurs. ● Patho: Possible causes ○ K loosing diuretics, thiazides and loop diuretics ○ Med: corticosteroids, sodium penicillin, and amphotericin hypokalemia ○ GI loss: vomiting and gastric suction ■ frequently lead to , because potassium is lost when gastric fluid is lost and because potassium is lost through the kidneys in response to metabolic alkalosis. ○ More GI losses: diarrhea, prolonged intestinal suctioning, recent ileostomy, and villous adenoma (a tumor of the intestinal tract characterized by excretion of potassium-rich mucus). ○ Respiratory or metabolic alkalosis ■ promotes the transcellular shift of potassium ● hydrogen ions move out of the cells in alkalotic states to help correct the high pH, and potassium ions move in to maintain an electrically neutral state (see later discussion of acid–base balance). ○ Hyperaldosteronism (retain Na and H2O, excrete K+ ○ Persistent insulin hypersecretion, ■ Insulin promotes the entry of potassium into skeletal muscle and hepatic cells. ■ Can happen in patients receiving high-carbohydrate parenteral nutrition. ○ Patients who don't eat a normal diet: geriatric patient, alcoholism or anorexia nervosa, bulimia too especially with vomiting. ● Clinical Manifestations: ○ Severe hypokalemia causes death, due to cardiac and respiratory attack. ○ Signs appear < 3 mEq/L ○ Prolonged causes inability of kidneys to concentrate urine, ■ Get dilute urine (polyuria, nocturia) ○ Increased Thirst ○ Low potassium leads to decrease in insulin and thus, glucose intolerance. ● Assessment/Diagnostic findings: ○ Fatigue, ○ anorexia, ○ muscle weakness, ○ decreased bowel motility, ○ paresthesias, ○ ECG changes = flat T waves or inverted, and.or ELEVATED U segment ■ Suggests dysrhythmias, ischemia and depressed ST segments ○ Increased sensitivity to digitalis, increasing risk of digitalis toxicity ○ Metabolic alkalosis ○ If unknown: 24-hour urinary potassium excretion test can be performed ■ distinguishes between renal and extrarenal loss. ● Urinary potassium excretion exceeding 20 mEq/day with hypokalemia suggests that renal potassium loss is the cause. ● Medical Management: ○ Daily diet or oral supplements ■ Dietary intake of potassium in the average adult is 50 to 100 mEq/day. ● Foods high in potassium include most fruits and vegetables, legumes, whole grains, milk, and meat ○ IV replacement therapy ■ Must be corrected daily: administration of 40 to 80 mEq/day of potassium is adequate in the adult if there are no abnormal losses of potassium. ■ Mandatory for severe: 2 mEq/L ● ● ■ KCl, or potassium acetate or potassium phosphate Nursing Management: ○ Oral route is best ■ BUT Oral potassium supplements can produce small bowel lesions; therefore, the patient must be assessed for and cautioned about abdominal distention, pain, or GI bleeding. ○ Special care if they are older, ■ have lower lean body mass and total-body potassium levels and therefore lower potassium requirements ■ physiologic loss of renal function with advancing years, potassium may be retained more readily in older than in younger people. ○ IV K replacement ■ only after adequate urine output has been established. ● If urine decreases to less than 20 mL / hr for 2 hrs STOP infusion bec. ○ Oliguria can cause the serum potassium concentration to rise dangerously ■ ONLY done with extreme caution using an infusion pump with the patient monitored by continuous ECG ■ Monitor renal fxn by BUN and creatine ■ Potassium is never given by IV push or intramuscularly to avoid replacing potassium too quickly. IV potassium must be given using an infusion pump. ○ Prevention ○ Eat high K+ foods ■ Banana, melon, citrus fruits, fresh/frozen veg, lean meats, whole grains, milk. ○ If caused by use of laxatives or diuretics, educate patient ○ Carefully monitor I&O ■ 40 mEq of potassium is lost for every liter of urine output. ○ Monitor blood gas values Potassium excess (hyperkalemia). TIGHT AND CONTRACTED patients > 5 mEq/L Rare in pat. With norm renal fxn Older adults high risk o increased risk of hyperkalemia due to decreases in renin and aldosterone as well as an increased number of comorbid cardiac conditions often caused by iatrogenic (treatment-induced) causes less common than hypo but more dangerous bec cardiac arrest!!!! ● Patho: o decreased renal excretion of potassium § kidney injury, in those whom potassium levels increase as a result of infection or excessive intake of potassium in food or medications o rapid administration of potassium ○ ○ ○ ○ ○ ○ ○ ○ ○ o movement of potassium from the ICF compartment to the ECF compartment Hyperaldosteronism Addison’s disease ■ Bec deficient adrenal hormones lead to sodium loss and potassium retention. MEDICATIONS are very common causes: ■ KCL, heparin, ACE inhib., NSAID, beta blockers, cyclosporine, tacrolimus (Prograf), and potassium-sparing diuretics Acute and Chronic kidney disease: ■ GFR 10-20% less normal Improper K+ supp. Use Aged blood into patients with impaired renal FXN: ■ Aged (stored) blood should not be given to patients with impaired renal function, because the serum potassium concentration of stored blood increases due to red blood cell deterioration. Acidosis: ■ K+ out of cells into ECF, while H+ hydrogen ions move into the cell to buffer pH Tissue trauma: ■ Burns, crushing injuries, severe infections, lysises of cells with chemo cause increased K+ levels Pseudohyperkalemia: ■ improper collection or transport of a blood sample, ■ a traumatic venipuncture, ■ use of a tight tourniquet around an exercising extremity while drawing a blood sample, ● producing hemolysis of the sample before analysis, ■ Leukocytosis & thrombocytosis ■ Drawing blood above site of K+ infusion ■ familial pseudohyperkalemia, ● which potassium leaks out of the red blood cells while the blood is awaiting analysis ■ WHYYYYY elevated levels in the absence of clinical manifestations (e.g., normal ECG) should be verified by retesting. ● Clinical Manifestations ○ MOST important effect is its effect on MYOCARDIUM ■ At > 6 mEq/L, EKG has peaked, narrow T waves; ST-segment depression; and a shortened QT interval. ■ If the serum potassium level continues to increase, the PR interval becomes prolonged and is followed by disappearance of the P waves. ■ Finally, there is decomposition and widening of the QRS complex (see Fig. 13-6). ● Ventricular dysrhythmias and cardiac arrest may occur ○ Hypotension, bradycardic ○ Diarrhea and hyperactive bowel sounds ■ paralytic ileus, ○ Paralysis in extremities ● ● ○ Paresthesia ○ Increased DTR, deep tendon reflexes ○ Muscle weakness Assessment and Diagnostic findings: ○ Look at: ■ serum K+ levels ■ ECG changes ■ ABG analysis Medical Management: ○ Check EKG, if shortened repolarization and peaked T waves → ○ Repeat serum K+ lab ○ Nonacute sit: ■ Dietary restrict K+ and K+ meds ○ Administration, either orally or by retention enema, of cation exchange resins (e.g., sodium polystyrene sulfonate [Kayexalate]) may be necessary ■ Not for patient with a paralytic ileus, intestinal perforation can occur ● Kayexalate binds with other cations in the GI tract and contributes to the development of hypomagnesemia and hypocalcemia; ○ it may also cause sodium retention and fluid overload and should be used with caution in patients with heart failure ○ EMERGENCY Therapy: ■ May be necessary to administer IV calcium gluconate ● Within minutes the calcium antagonizes the action of hyperkalemia on the heart but does not reduce the serum potassium concentration. ○ Calcium chloride and calcium gluconate are not interchangeable; calcium gluconate contains 4.5 mEq of calcium, and calcium chloride contains 13.6 mEq of calcium ● EXTRA precautions if patient has received an accelerated dose of a digitalis-based cardiac glycoside to reach a desired serum digitalis level rapidly as parenteral administration of calcium sensitizes the heart to digitalis and may precipitate digitalis toxicity. ■ Monitor BP: ● Hypotension is a risk from the rapid IV ■ Continuously monitor EKG ● If become bradycardic STOP infusion ■ Sodium Bicarb may be given in severe metabolic acidosis ● This alkalinizes the plasma, shift potassium into the cells, and furnish sodium to antagonize the cardiac effects of potassium. ■ Watch for FVE and hyperkalemia ■ Can also receive dosage of IV reg insulin ● Moves K+ into cells ■ Loop diuretics: ● Removes Na+, water and K+ ■ Beta 2 agonists ● Ex: Albuterol ● ● ● ● moves potassium into the cells and may be used in the absence of ischemic cardiac disease. ■ OR peritoneal dialysis, hemodialysis, or other forms of renal replacement therapy Nursing Management: ○ For pat. At risk→ nurse manages: ■ I&O ■ Observes signs of muscle weakness and dysrhythmias ■ During VS: apical pulse should be taken ■ Look for presence of paresthesias and GI symptoms such as nausea and intestinal colic ■ LABS: ● Serum potassium levels, as well as BUN, creatinine, glucose, and arterial blood gas values Prevention ○ Encourage K+ diet restriction ■ include many fruits and vegetables, legumes, whole-grain breads, lean meat, milk, eggs, coffee, tea, and cocoa VS ● Correcting it: ○ IV route ■ monitor potassium solutions closely. Particular attention is paid to the solution’s concentration and rate of administration. IV administration is via an infusion pump 5. Describe the cause, clinical manifestations, management, and nursing interventions for the following imbalances: calcium deficit (hypocalcemia) and calcium excess (hypercalcemia), magnesium deficit (hypomagnesemia) and magnesium excess (hypermagnesemia), phosphorus deficit (hypophosphatemia) and phosphorus excess (hyperphosphatemia), and chloride deficit (hypochloremia) and chloride excess (hyperchloremia). HYPOCALCEMIA (<than 8.6 mg/dL [2.15 mmol/L]) - Pt may have a total-body calcium deficit (as in osteoporosis) but a normal serum calcium level. Older adults and those with disabilities, that spend an increased amount of time in bed, have an increased risk of hypocalcemia, because bed rest increases bone resorption. Causes - - Primary hypoparathyroidism Surgical hypoparathyroidism (more common); associated with thyroid and parathyroid surgery Can occur after radical neck dissection Transient hypocalcemia can occur with massive administration of citrated blood (i.e., massive hemorrhage and shock), because citrate can combine with ionized calcium and temporarily remove it from the circulation. Pancreatitis breaks down proteins & fats. This can cause Ca2+ ions to combine w/ fatty acids from lipolysis, forming soap. May be related to excessive secretion of glucagon from the inflamed pancreas, which results in increased secretion of calcitonin. Kidney injury, because pts with kidney injuries frequently have hyperphosphatemia. That in turn causes a reciprocal drop in the serum calcium level. Inadequate vitamin D consumption, magnesium deficiency, medullary thyroid carcinoma, low serum albumin levels, alkalosis, and alcohol abuse Medications predisposing to hypocalcemia include aluminum-containing antacids, aminoglycosides, caffeine, cisplatin, corticosteroids, mithramycin, phosphates, isoniazid, loop diuretics, and proton pump inhibitors Clinical manifestations - - s/s are caused by spontaneous discharges of both sensory & motor fibers in peripheral nerves Chvostek sign - Trousseau sign - Seizures due to increased irritability of CNS & PNS. Mental changes such as depression, impaired memory, confusion, delirium, and hallucinations A prolonged QT interval is seen on the ECG due to prolongation of the ST segment, and torsades de pointes, a type of ventricular tachycardia, may occur. Respiratory effects with decreasing calcium include dyspnea and laryngospasm. S/S of chronic hypocalcemia include hyperactive bowel sounds, dry and brittle hair and nails, and abnormal clotting. Osteoporosis is associated with prolonged low intake of calcium and represents a total-body calcium deficit, even though serum calcium levels are usually normal. Assessment & diagnostic findings -When evaluating serum calcium levels, the serum albumin level and the arterial pH must also be considered. When serum albumin abnormal: For every decrease in serum albumin of 1 g/dL below 4 g/dL, the total serum calcium level is underestimated by approximately 0.8 mg/dL Management - - Emergency Pharmacologic Therapy - IV administration of Ca2+ salt - These salts are either calcium chloride or calcium gluconate (more common). Although calcium chloride yields more Ca2+ , it is more irritating. - IV admin is dangerous for pts receiving digitalis-derived meds & can lead to digitalis toxicity - 0.9% NaCl solution & solutions containing phosphates or bicarbonates shouldn’t be used - Calcium replacement can cause postural hypotension; therefore, the patient is kept in bed during IV infusion, and blood pressure is monitored Nutrition therapy - Vit D (increased calcium absorption from the GI tract) - Aluminum hydroxide, calcium acetate, or calcium carbonate antacids may be prescribed to decrease elevated phosphorus levels for pts with chronic kidney disease. - Increasing the dietary intake of Ca2+ to at least 1000 to 1500 mg/day in the adult is recommended Calcium-containing foods include milk products; green, leafy vegetables; canned salmon; canned sardines; and fresh oysters. Calcium supplements must be given in divided doses of no higher than 500 mg Nursing interventions - Assess for hypocalcemia in at-risk patients. Initiate seizure precautions if hypocalcemia is severe. The status of the airway is closely monitored, because laryngeal stridor can occur Educate the pt about foods that are rich in calcium or consider calcium supplements. Such supplements should be taken in divided doses with meals. Alcohol and caffeine in high doses inhibit calcium absorption, and moderate cigarette smoking increases urinary calcium excretion. Educate pt to avoid the overuse of laxatives and antacids that contain phosphorus, because their use decreases calcium absorption. HYPERCALCEMIA (>10.2 mg/dL [2.6 mmol/L]) - Very dangerous Mortality rate of 50% if not treated immediately Causes - Common causes = malignancies & hyperparathyroidism (excessive PTH secretion) Lost bone mineral during immobilization Thiazide diuretics can cause slight elevation of serum Ca2+ Vit A & D intoxication, chronic lithium use, theophylline toxicity Calcium levels are inversely related to phosphorus levels. Clinical manifestations - s/s are proportional to the degree of elevation of the serum calcium level. Severity increases with increases in Ca2+ levels. Hypercalcemia reduces neuromuscular excitability because it suppresses activity at the myoneural junction. Can aggravate digitalis toxicity Hypercalcemic crisis (17 mg/dL (4.3 mmol/L) or higher) Severe thirst, polyuria often present Muscle weakness, intractable nausea, abdominal cramps, severe constipation, diarrhea, peptic ulcer symptoms, and bone pain. Lethargy, confusion, and coma may also occur. Dangerous! Can result in cardiac arrest. Calcitonin is used for emergency treatment. Management -Treatment targets to decrease serum calcium level or treat the underlying disease causing it - Giving fluids to dilute serum calcium and promote its excretion by the kidneys, mobilizing the patient, and restricting dietary calcium intake. - Administering IV phosphate can cause a reciprocal drop in serum calcium. - Furosemide increases Ca2+ excretion - Calcitonin reduces bone resorption, increases the deposition of calcium and phosphorus in the bones, and increases urinary excretion of calcium and phosphorus. - For pts with cancer, treatment is directed at controlling the condition by surgery, chemotherapy, or radiation therapy. Corticosteroids may be used in some cases. Assessment & diagnostic findings - - Cardiovascular changes may include a variety of dysrhythmias (e.g., heart blocks) and shortening of the QT interval and ST segment. The double-antibody PTH test may be used to differentiate between primary hyperparathyroidism and malignancy as a cause of hypercalcemia: PTH levels are increased in primary or secondary hyperparathyroidism and suppressed in malignancy. X-rays may reveal bone changes if the patient has hypercalcemia secondary to a malignancy, bone cavitations, or urinary calculi. Urine calcium can be normal or elevated in hyperparathyroidism and hypercalcemia caused by malignancy. Nursing interventions - Increasing patient mobility and encouraging fluids can help prevent hypercalcemia, or at least minimize its severity. Fluids containing sodium should be given unless contraindicated, because sodium assists with calcium excretion. Adequate fiber in the diet is encouraged to offset the tendency for constipation. Assess pts for signs of digitalis toxicity If pts show altered mental status, let family know that is can be reversed with treatment Because ECG changes can occur, the cardiac rate and rhythm are monitored for any abnormalities. Pts are encouraged to drink 2.8 to 3.8 L (3 to 4 quarts) of fluid daily Hospitalized pts, outpatients & those who receive home care should be taught about the importance of ambulating frequently. HYPOMAGNESEMIA (<1.3 mg/dL [0.62 mmol/L]) - Frequently associated w/ hypokalemia & hypocalcemia Mg2+ is similar to Ca2+ in two aspects: - 1. it is the ionized fraction of Mg2+ that is primarily involved in neuromuscular activity and other physiologic processes - 2. Mg levels should be evaluated in combination with albumin levels. Causes - - GI tract is an important route of loss for Mg2+. It can happen with nasogastric suction, diarrheas and fistulas - Diarrheas & fistulas cause more sever hypomagnesemia. - Hypomagnesemia from nasogastric suction is low but condition exacerbates if losses are prolonged & Mg2+ is not replaced Disruption in small bowel fxn (small bowel is a major site for Mg absorption) Can occur from withdrawal from alcohol and administration of tube feedings or parenteral nutrition. Chronic alcohol abuse Parenteral or enteral feedings that are low in Mg content Often occurs in diabetic ketoacidosis, secondary to increased renal excretion during osmotic diuresis and shifting of magnesium into the cells with insulin therapy. Administration of aminoglycosides, cyclosporine, cisplatin, diuretics, digitalis, and amphotericin, as well as the rapid administration of citrated blood, especially to patients with renal or hepatic disease. Clinical manifestations -Some clinical manifestations are due to direct changes in Mg2+ while others are due to secondary changes in K+ and Ca2+ levels. -s/s don’t occur until levels drop to less than 1.8 mEq/L (0.75 mmol/L). -Chvostek and Trousseau signs occur, in part, because of accompanying hypocalcemia - Alterations in psychological status --> pathy, depressed mood, apprehension, and extreme agitation, ataxia, dizziness, insomnia, and confusion. At times, delirium, auditory or visual hallucinations, and frank psychoses may occur. - Magnesium deficiency can disturb the ECG by prolonging the QRS, depressing the ST segment, and predisposing to cardiac dysrhythmias -Increased susceptibility to digitalis toxicity is associated with low serum magnesium levels. -Important, because patients receiving digoxin are also likely to be receiving diuretic therapy, predisposing them to renal loss of magnesium. -Concurrent hypokalemia and hypocalcemia must be addressed in addition to hypomagnesemia. These electrolyte disturbances are difficult to correct until magnesium has been repleted. Assessment & diagnostic findings - Lab analysis Urine magnesium can help identify the cause of magnesium depletion after a loading dose of magnesium sulfate is given. Diagnostic techniques (nuclear magnetic resonance spectroscopy and the ion-selective electrode) Management -Mild Mg deficiency can be corrected by diet alone. Eat foods high in green leafy vegetables, nuts, seeds, legumes, whole grains, seafood, peanut butter, and cocoa. -If necessary, Mg salts can be given orally; can cause diarrhea -Pts receiving parenteral nutrition need Mg in the IV solution -Vital signs must be assessed frequently during magnesium administration to detect changes in cardiac rate or rhythm, hypotension, and respiratory distress. -Monitoring urine output is essential before, during, and after magnesium administration; the physician is notified if urine volume decreases to less than 100 mL over 4 hours. Nursing interventions -Monitor pts at risk for hypomagnesemia & monitor them closely -Monitor pts receiving digitalis closely -If hypomagnesemia is severe, take seizure precautions -Observe for any dysphagia or confusion -Educate pts about magnesium-rich foods, including green vegetables, nuts, legumes, bananas, and oranges HYPERMAGNESEMIA - > 3.0 mg/dL [1.25 mmol/L]) Rare! Cuz kidneys excrete Mg efficiently Causes -kidney injury (most common) -Can occur in pts w/ untreated diabetic ketoacidosis - Excessive magnesium given to treat hypertension of pregnancy or to treat hypomagnesemia. -Adrenocortical insufficiency, Addison disease, or Hypothermia -Excessive use of magnesium-based antacids or laxatives and meds that decrease GI motility, including opioids and anticholinergics, can also increase serum Mg levels. -Decreased elimination of magnesium or its increased absorption due to intestinal hypomotility -Lithium intoxication -Extensive soft tissue injury or necrosis Clinical manifestations -Acute rise in Mg --> depressed CNS & peripheral neuromuscular junction -Coma, atrioventricular heart block, and cardiac arrest can occur -Can result in platelet clumping and delayed thrombin formation. Management -Avoid giving Mg to pts w/ kidney injuries -Carefully monitor pts receiving Mg salts -In severe cases, all parenteral and oral magnesium salts are discontinued. - In emergencies, such as respiratory depression or defective cardiac conduction, ventilatory support and IV calcium gluconate are indicated. -hemodialysis with a magnesium-free dialysate can reduce the serum magnesium to a safe level within hours. - Administration of loop diuretics and sodium chloride or lactated Ringer IV solution increases Mg excretion in pts with adequate renal function. -IV calcium gluconate antagonizes the cardiovascular and neuromuscular effects of magnesium. Assessment & diagnostic findings -lab analysis = Mg is > than 3.0 mg/dL (1.25 mmol/L) -Increased potassium and calcium are present concurrently. -ECG findings may include a prolonged PR interval, tall T waves, a widened QRS, and a prolonged QT interval, as well as an atrioventricular block Nursing interventions -If hypomagnesem ia is suspected, monitor vitals & note any hypotension or shallow respirations -Observe for decreased deep tendon reflexes (DTRs) and changes in the level of consciousness -Don’t give Mg containing meds to pts w/ kidney injury or impaired renal fxns. HYPOPHOSPHATEMIA <2.5 mg/dL (0.8 mmol/L) Causes -Even if pt has hypophosphatemia, their total-body phosphorous stores can still be normal. Conversely, even is a pt has phosphorus deficiency in lean tissues, they may not always have hypophosphatemia. - Excessive intake of simple carbohydrates - Specifically, when administering calories to protein-calorie malnutrition pts. Ex: anorexia, alcoholism etc - Heat stroke, prolonged intense hyperventilation, alcohol withdrawal, poor dietary intake, diabetic ketoacidosis, respiratory alkalosis, hepatic encephalopathy, and major thermal burns. - Low magnesium levels, low potassium levels, and hyperparathyroidism related to increased urinary losses of phosphorus contribute to hypophosphatemia. - Acute volume expansion, osmotic diuresis, the use of carbonic anhydrase inhibitors (acetazolamide [Diamox]), and some malignancies. -Excess phosphorus binding by antacids - Chronic diarrhea, Crohn’s disease, vitamin D deficiency, anorexia, alcoholism, and malabsorption - Deficiency of vitamin D Clinical manifestations -Most s/s of hypophosphatemia are due to deficiency of ATP, 2,3-diphosphoglycerate, or both -ATP deficiency --> impaired cellular energy resources. diphosphoglycerate deficiency impairs oxygen delivery to tissues --> wide range of neurologic manifestations. -muscle weakness, muscle pain, acute rhabdomyolysis (breakdown of skeletal muscle). -predispose a person to insulin resistance and thus hyperglycemia. -bruising and bleeding from platelet dysfunction. Assessment & diagnostic findings -lab analysis = less than 2.5 mg/dL (0.80 mmol/L) of phosphate -Keep in mind that glucose or insulin administration causes a slight decrease in the serum phosphorus level Management -Prevention is the goal!! -For pts at risk, closely monitor phosphate levels & correct asap. - Make sure there’s enough phosphate in parenteral/enteral feedings -Severe hypophosphatemia is dangerous. Needs immediate attention. -Administered via IV only for pts whose phosphate levels are <1 mg/dL (0.3 mmol/L) and whose GI tract is not functioning. Otherwise give oral phosphorus replacement. Nursing interventions -Malnourished pts receiving parenteral nutrition are at risk when calories are introduced too aggressively. So gradually introduce the solution -For pts that already have hypophosphatemia, goal is to prevent infection cuz it can alter granulocytes. - Mild hypophosphatemia --> encourage pts abt foods such as milk and milk products, organ meats, nuts, fish, poultry, and whole grains -Moderate hypophosphatemia -->supplements such as Neutra-Phos capsules, K-Phos, and Fleet Phospho-Soda may be prescribed. HYPERPHOSPHATEMIA >4.5 mg/dL (1.45 mmol/L). Causes -kidney injury (most common) - increased intake, decreased output, or a shift from the intracellular to extracellular space -excessive vitamin D intake, administration of total parenteral nutrition, chemotherapy for neoplastic disease, hypoparathyroidism, metabolic or respiratory acidosis, diabetic ketoacidosis, acute hemolysis, high phosphate intake, profound muscle necrosis, and increased phosphorus absorption **Primary complication of hyperphosphatemia is metastatic calcification (soft tissue, joints, and arteries) Clinical manifestations -Most s/s are due to decreased Ca2+ levels & soft calcifications -major short term consequence = tetany -reciprocal relationship b/w Ca and P. High P --> Low Ca. Low P --> High Ca -Major long term problem = soft tissue calcification -mainly occurs in pts with reduced GFR -Decrease urine output, impaired vision, palpitations Assessment & diagnostic findings -lab analysis where serum P level is > 4.5 mg/dL (1.5 mmol/L) -Serum Ca level is useful for diagnosing primary problem & assessing treatments -X-Rays may show abnormal bone development PTH levels decrease in hyperparathyroidism BUN & creatinine levels help indicate renal fxn Management -When possible, treat underlying primary disroders -Calcitriol (Vit D), Ca-binding antacids & Amphojel are used for treatments -Restriction of dietary phosphate, forced diuresis with a loop diuretic, volume replacement with saline, and dialysis -Surgery for removal of large deposits of Ca & P Nursing interventions -For low P diet, avoid foods like hard cheeses, cream, nuts, meats, whole-grain cereals, dried fruits, dried vegetables, kidneys, sardines, sweetbreads, and foods made with milk. -educate pt to avoid phosphate-containing laxatives and enemas. -educate pt about recognizing signs of impending hypocalcemia and monitoring for changes in urine output HYPOCHLOREMIA Causes -GI tube drainage, gastric suctioning, gastric surgery, and severe vomiting and diarrhea. -Administration of chloride-deficient IV solutions, low sodium intake, decreased serum sodium levels, metabolic alkalosis, massive blood transfusions, diuretic therapy, burns, and fever -Administration of aldosterone, ACTH, corticosteroids, bicarbonate, or laxatives Complication: As Cl decreases, Na and HCO3-(bicarbonate) ions are retained by the kidney to balance the loss. HCO3- accumulates in the ECF --> increase in pH -->hypochloremic metabolic alkalosis. Clinical manifestations -metabolic alkalosis causes high pH & serum HCO3-. To compensate, the PaCO2 in arterial blood increases to 50mmHg --> hyperexcitability of muscles, tetany, hyperactive DTRs, weakness, twitching, and muscle cramps -Hypokalemia can cause hypochloremia --> cardiac dysrhythmias -Hypochloremia can parallel Na+ levels --> water excess & low Na+ --> hyponatremia --> may lead to seizures & coma Assessment & diagnostic findings -serum Cl < 97 mEq/L (97 mmol/L). -Na+ & K+ levels are also monitored -ABG analysis helps determine acid-base imbalance (usually metabolic alkalosis) -Urine Cl- level measured (usually decreases) Management -Correct the primary cause & the contributing acid/base/electrolyte imbalance -IV saline is given to replace the Cl-Current diuretics may be discontinued or a different one is prescribed -Ammonium chloride is prescribed for metabolic alkalosis; should be avoided in pts w/ liver or renal issues. Nursing interventions -Nonitor Pt’s I&O, arterial blood gas values, and serum electrolyte levels. -Changes in the patient’s level of consciousness and muscle strength and movement are reported to the primary provider promptly. -Vital signs are monitored, and respiratory assessment is carried out frequently. -Educate the patient about foods with high chloride content, which include tomato juice, bananas, dates, eggs, cheese, milk, salty broth, canned vegetables, and processed meats. -Tell pt to avoid drinking free water (water w/o electrolytes) HYPERCHLOREMIA Causes -Complication: Hypernatremia, bicarbonate loss, and metabolic acidosis - iatrogenically induced hyperchloremic metabolic acidosis due to excess Cl- administration, saline solution or lactated ringer solution -Loss of HCO3- via kidney/GI tract --> increase in Cl- (in the form of acidifying salts) --> acidosis -Head trauma, increased perspiration, excess adrenocortical hormone production, and decreased GFR Clinical manifestations -s/s are same as those of metabolic acidosis: hypervolemia, hypernatremia -Tachypnea, weakness, lethargy, deep and rapid respirations, diminished cognitive ability, and hypertension untreated, hyperchloremia --> decrease in cardiac output, dysrhythmias, and coma High Cl- --> high Na+ & fluid retention Assessment & diagnostic findings Cl- is > 108 mEq/L (108 mmol/L) Na+ is > 145 mEq/L (145 mmol/L) pH is less than 7.35 serum bicarbonate level is less than 22 mEq/L (22 mmol/L) Urine chloride excretion increases. Management -Correct underlying cause -restore electrolyte, fluid, and acid–base balance via hypotonic IV solutions -Lactated ringer solution or IV Sodium bicarbonate to correct acidosis -Diuretics to eliminate Cl-Sodium, chloride, and fluids are restricted. Nursing interventions ● ● ● Monitoring vital signs, arterial blood gas values, and I&O Assessment findings related to respiratory, neurologic, and cardiac systems. Changes are discussed with primary provider Educate the patient about the diet that should be followed to manage hyperchloremia and maintain adequate hydration. 6. Explain the roles of the lungs, kidneys, and chemical buffers in maintaining acid–base balance. -Identification of the specific acid–base imbalance is important in ascertaining the underlying cause of the disorder and determining appropriate treatment. -Homeostatic pH is 7.35-7.45 - pH is a measure of the number of H+ in a solution - more H+, more acidic, the Lower pH -Less H+, more alkalinic, the higher the pH -pH is maintained by: 1) Lungs maintain acid-base balance by: a) Under control of the medulla, control CO2 and therefore carbonic acid concentration. b) VERY FAST RESPONSE i) CO2 is a potential acid; when dissolved in water, it becomes carbonic acid (CO2 + H2O = H2CO3). (1) Do so by increasing or decreasing ventilation (a) EX: ↑↑ in PaCO2 → increases respiration (i) Oxygen also stim resp. But not as much as CO2 ii) EX: metabolic acidosis, RR ↑, ↑ elimination of CO2 iii) Alkalosis: RR ↓, and ↑ retained CO2 2) Kidneys maintain acid-base balance by: a) Regulate bicarb level in ECF b) VERY SLOW i) Can regenerate bicarb ions are reabsorbed from renal tubular cells ii) Excrete H+ ions, and conserve bicarb in respiratory acidosis and most cases of metabolic acidosis, iii) However in resp, and metabolic alkalosis: they retain H+ and excrete bicarb (1) Can do this of course unless the kidney is injured and therefore the cause of metabolic acidosis. 3) Chemical Buffers maintain acid-base balance by: a) Buffer systems prevent major changes in the pH of body fluids by removing or releasing H+, and can do so quickly i) The bicarbonate–carbonic acid buffer system, is the body’s major extracellular buffer system (1) assessed when arterial blood gases are measured. (2) There are 20 parts of bicarbonate (HCO3−) to 1 part of carbonic acid (H2CO3). 20:1 (a) When altered, the pH changes. (i) It is the ratio of HCO3− to H2CO3 that is important in maintaining pH, not absolute values. (3) CO2 is a potential acid; when dissolved in water, it becomes carbonic acid (CO2 + H2O = H2CO3). (a) ↑CO2 --> ↑carbonic acid content is also increased ii) Inorganic phosphates and the plasma proteins (1) Less impt ECF buffers iii) Proteins, organic and inorganic phosphates, and, in red blood cells, hemoglobin (1) Intracellular buffers 7. Compare metabolic acidosis and alkalosis with regard to causes, clinical manifestations, diagnosis, and management. METABOLIC ACIDOSIS (base bicarbonate deficit) ● ● ● characterized by low pH (high H+) & low plasma HCO3can be produced by gain of H+ or loss of HCO32 forms: high anion gap acidosis and normal anion gap acidosis ○ Anion gap = (sum of all cations) - (sum of all anions) ○ Measuring the anion gap is important for analyzing acid-base disorders ○ ○ ○ ● The 2nd equation is used more often than the first cuz K+ levels in plasma are usually low the anion gap reflects normally unmeasured anions (phosphates, sulfates, and proteins) in plasma that increase the anion gap by replacing bicarbonate. An anion gap > than 16 mEq (16 mmol/L) suggests excessive accumulation of unmeasured anions and would indicate high anion gap metabolic acidosis as the type. CAUSES ● ● Causes of normal anion gap (a.k.a hypochloremic acidosis) ○ direct loss of HCO3-, as in diarrhea, lower intestinal fistulas, ureterostomies, and the use of diuretics; early renal insufficiency; excessive administration of chloride; administration of parenteral nutrition without bicarbonate or bicarbonate-producing solutes Causes of high anion gap: ○ Excessive accumulation of fluid due to ketoacidosis, lactic acidosis, the late phase of salicylate poisoning, uremia, methanol or ethylene glycol toxicity, and ketoacidosis with starvation CLINICAL MANIFESTATIONS ● ● ● ● s/s vary with severity but include headache, confusion, drowsiness, increased respiratory rate and depth, nausea, and vomiting Peripheral vasodilation and decreased cardiac output occur when the pH drops to less than 7. decreased blood pressure, cold and clammy skin, dysrhythmias, and shock Complication: Chronic metabolic acidosis is usually seen with chronic kidney disease. DIAGNOSIS ● ● ● ● ● ABG analysis will indicate low HCO3- & low pH (imp indicators) Hyperkalemia may occur. When acidosis occurs, hypokalemia may occur Calculation of anion gap Hyperventilation lowers CO2 level as compensatory action ECG detects dysrhythmias caused by increased K+ MANAGEMENT ● ● ● ● Treatment targets correcting metabolic imbalance ○ Ex: if acidosis is caused by hyperchloremia, goal is to eliminate source of ClHCO3- is only given when necessary. If given during cardiac arrest --> paradoxical intracellular acidosis Monitor serum K+ level closely, and hypokalemia is corrected as acidosis is reversed. In chronic metabolic acidosis, low Ca2+ levels are treated before treating the acidosis to prevent tetany ○ Alkalizing agents may be given ○ Treatment can include hemodialysis or peritoneal dialysis. METABOLIC ALKALOSIS (Base bicarbonate excess) -High pH & high plasma HCO3-result of excess HCO3- or loss of H+ CAUSES ● ● ● ● ● ● Vomiting or gastric suction (common cause) Pyloric Stenosis Predisposing factors: loss of potassium (diuretics), ACTH secretion (hyperaldosteronism and Cushing syndrome). Hypokalemia causes alkalosis in 2 ways: ○ 1. The kidneys conserve K+ --> increase in H+ excretion ○ 2. Cellular K+ moves out of the cells into the ECF in an attempt to maintain near-normal serum levels. As K+ leaves, H+ must enter cells to maintain electroneutrality. Excessive alkali ingestion from antacids containing bicarbonate or from the use of sodium bicarbonate during cardiopulmonary resuscitation Chronic metabolic alkalosis causes: ○ long-term diuretic therapy, villous adenoma, external drainage of gastric fluids, significant K+ depletion, cystic fibrosis, and the chronic ingestion of milk and calcium carbonate. CLINICAL MANIFESTATIONS ● ● ● ● ● s/s are mainly due to decreased calcium ionization tingling of the fingers and toes, dizziness, and hypertonic muscles Symptoms of hypocalcemia are often the predominant symptoms of alkalosis. Depressed respirations, atrial tachycardia, decreased motility and paralytic ileus. s/s for chronic metabolic alkalosis is the same acute metabolic alkalosis. ○ As K+ goes down, frequent premature ventricular contractions or U waves are seen on the ECG. DIAGNOSIS ● ● ● ● ABG analysis will show pH > 7.45 & HCO3- > 26 mEq/L PaCO2 increases (compensatory mechanism by lungs) ○ More common in semiconscious, unconscious, or debilitated patients than in alert patients. These pts may also develop hypoventilation due to compensation Hypokalemia Urine Cl- levels ○ help to differentiate between vomiting, diuretic therapy, and excessive adrenocorticosteroid secretion as the cause of the metabolic alkalosis. ○ The urine chloride concentration should be less than 15 mEq/L when decreased chloride levels and hypovolemia occur. MANAGEMENT ● ● ● ● ● ● Goal is to treat the primary acid-base disorder Monitor Pt’s I & Os. Administer sodium chloride fluids (to restore normal fluid volume) For hypokalemia, potassium is given as KCl (to replace both K+ and Cl− losses). H2 receptor antagonists, such as cimetidine (Tagamet), reduce the production of gastric HCl --> lowers metabolic alkalosis associated with gastric suction. Carbonic anhydrase inhibitors (for pts who cannot tolerate rapid volume expansion. Ex: pts w/ heart failure). 7. Compare respiratory acidosis and alkalosis with regard to causes, clinical manifestations, diagnosis, and management. Respiratory Acidosis: - Low pH < 7.35 PaCO2 >42 Respiratory problem with inadequate excretion of CO2 and inadequate ventilation causing increased carbonic acid levels in the body. With chronic respiratory acidosis body can compensate and be asymptomatic: - COPD Cause: - - - Occurs in emergency situations, such as: - Acute pulmonary edema - Aspiration of a foreign object - Atelectasis - Pneumothorax - Overdose of sedatives Some non emergency situations: - Sleep apnea associated with obesity, administration of O2 to a pat with chronic hypercapnia - Severe pneumonia - Acture respiratory distress syndrome. Also in diseases that impair the respiratory muscles: - Muscular dystrophy multiple sclerosis, myasthenia gravis, and Guillain–Barré syndrome Clinical manifestations: - - S/S vary: Sudden hypercapnia causes: - Increased pulse - Increased RR - Increased BP - Mental cloudiness or confusion - Fullness feeling in head - Decreased level of consciousness PaCO2 > 60 mm hg causes: cerebrovascular vasodilation and increased cerebral blood flow. Ventricular fibrillation may be the first sign of respiratory acidosis in anesthetized patients. Severe: intracranial pressure could increase, causing papilledema, and dilated conjunctival blood vessels. Hyperkalemia may result as the hydrogen concentration overwhelms the compensatory + mechanisms and H moves into cells, causing a shift of potassium out of the cell. Chronic causes: - Commonly asymptomatic if being compensated - If CO2 increases rapidly, cerebral vasodilation will increase the intracranial pressure, and cyanosis and tachypnea will develop. Diagnosis: - ABG = pH < 7.35 and PaCO2 > 42 mm Hg, and Bicarb with vary based on length of acidosis Management: - Improve ventilation, vary with the cause of inadequate ventilation Meds are used as indicated - Bronchodilators etc. Pulmonary hygiene measures are initiated, when necessary, to clear the respiratory tract of mucus and purulent drainage. Adequate hydration (2 to 3 L/day) is indicated to keep the mucous membranes moist and thereby facilitate the removal of secretion Supplemental O2 as needed Mechanical ventilation Have to decrease PaCO2 slowly Respiratory Alkalosis: : - pH > 7.45 PaCO2 < 35 Cause: - Hyperventilation: blows off too much CO2, and thus decreases plasma carbonic acid. - Can be caused by: - Anxiety - Hypoxemia - Ear;y phase of salicylate intoxication - Gram neg bacteria - Inapp. Ventilator settings - Chronic Alkalosis is from: - Chronic hypocapnia & Decreased serum bicarbonate levels - Chronic hepatic insufficiency and cerebral tumors are predisposing factors Clinical manifestations: - Light headedness: - Due to vasoconstriction and decreased cerebral BF Inability to concentrate Numbness & tingling - Due to decreased calcium ionization Sometimes Loss of consciousness Diagnosis: - In the acute state, the pH is elevated above normal as a result of a low PaCO2 and a normal bicarbonate level - When compromised the kidneys have had sufficient time to lower the bicarbonate level to a near-normal level Electrolytes: - Potassium will be low as the H+ is being pulled out of the cells and K+ pushed in - + Calcium will decrease, as severe alkalosis inhibits calcium ionization, resulting in carpopedal spasms and tetany; or decreased phosphate due to alkalosis, causing an increased uptake of phosphate by the cells Management - Depends on cause If anxiety, tell them to breathe slowly, or into a paper bag 8. Interpret arterial blood gas measurements. TABLE 13-1 Concentrations of Extracellular and Intracellular Electrolytes in Adults Extracellular Concentrations Electrolyte Conventional Units SI Units Intracellular Concentration Conventional Units SI Units (mmol/L) (mmol/L) Sodium 135–145 mEq/L 135–145 10–14 mEq/L 10–14 Potassium 3.5–5.0 mEq/L 3.5–5.0 140–150 mEq/L 140–150 Chloride 98–106 mEq/L 98–106 3–4 mEq/L 3–4 Bicarbonate 24–31 mEq/L 24–31 7–10 mEq/L 7–10 Calcium 8.5–10.5 mg/dL 2.1–2.6 <1 mEq/L <0.25 Phosphorus 2.5–4.5 mg/dL 0.8–1.45 Variable Variable 1.8–3.0 mg/dL 0.75–1.25 40 mEq/kgb 20 Magnesium ● ● Blood gas analysis is often used to identify the specific acid–base disturbance and the degree of compensation that has occurred. ○ based on an arterial blood sample; however, if an arterial sample cannot be obtained, a mixed venous sample may be used. The health history, physical examination, previous blood gas results, and serum electrolytes should always be part of the assessment used to determine the cause of the acid–base disorder GLOSSARY acidosis: an acid–base imbalance characterized by an increase in H+ concentration (decreased blood pH) (A low arterial pH due to reduced bicarbonate concentration is called metabolic acidosis; a low arterial pH due to increased PCO2 is called respiratory acidosis.) ascites: a type of edema in which fluid accumulates in the peritoneal cavity active transport: physiologic pump that moves fluid from an area of lower concentration to one of higher concentration; active transport requires adenosine triphosphate for energy alkalosis: an acid–base imbalance characterized by a reduction in H+ concentration (increased blood pH) (A high arterial pH with increased bicarbonate concentration is called metabolic alkalosis; a high arterial pH due to reduced PCO2 is called respiratory alkalosis.) diffusion: the process by which solutes move from an area of higher concentration to one of lower concentration; does not require expenditure of energy homeostasis: maintenance of a constant internal equilibrium in a biologic system that involves positive and negative feedback mechanisms hydrostatic pressure: the pressure created by the weight of fluid against the wall that contains it. In the body, hydrostatic pressure in blood vessels results from the weight of fluid itself and the force resulting from cardiac contraction. hypertonic solution: a solution with an osmolality higher than that of serum hypotonic solution: a solution with an osmolality lower than that of serum isotonic solution: a solution with the same osmolality as serum and other body fluids osmolality: the number of milliosmoles (the standard unit of osmotic pressure) per kilogram of solvent; expressed as milliosmoles per kilogram (mOsm/kg). (The term osmolality is used more often than osmolarity to evaluate serum and urine.) osmolarity: the number of milliosmoles (the standard unit of osmotic pressure) per liter of solution; expressed as milliosmoles per liter (mOsm/L); describes the concentration of solutes or dissolved particles osmosis: the process by which fluid moves across a semipermeable membrane from an area of low solute concentration to an area of high solute concentration; the process continues until the solute concentrations are equal on both sides of the membrane tonicity: fluid tension or the effect that osmotic pressure of a solution with impermeable solutes exerts on cell size because of water movement across the cell membrane USEFUL VIDEOS TO WATCH https://thepoint.lww.com/VitalSource/Engine/774963?showDebriefingLog=False https://thepoint.lww.com/VitalSource/Engine/774964?showDebriefingLog=False https://thepoint.lww.com/VitalSource/Engine/774965?showDebriefingLog=False