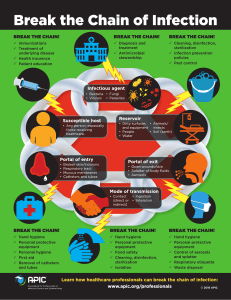
Viruses November 2020 What is a virus? An obligate intracellular parasite that consists of genomic RNA or DNA and a protein coat and/or envelope. Classification of viruses Overview of virus nucleic acid HIV-1 budding Virus assembly results in a burst of virus particles released Patterns of Infection Some reasons for chronic infection: Infection of immune privileged site, non-replicating within a cell, integration within host chromosome, Ebola virus replication Flaviviruses: Predominantly vector-borne Rathore & St. John Frontiers in Immunology 2020 Flavivirus Transmission Cycles Flavivirus Transmission Cycles Dengue transmission cycle Association of fetal congenital defects with Zika infection of pregnant mothers • The rates of microcephaly range from 1-30% in various studies. Brasil et al NEJM 2016 Pacheco et al NEJM 2016 • Microcephaly risk appears highest during the first trimester in humans. Cauchemez et al Lancet 2016 Johansson et al NEJM 2016 Cauchemez et al Lancet 2016 Zika has been shown to infect neural progenitor cells with direct inoculation into the brain Li et al Cell Stem Cell 2016 But the mechanisms of ZIKV vertical transmission into the fetus are unknown… Flavivirus replication Cell and tissue tropism influences the symptoms/course of infection Yellow Fever dengue jaundice Japanese encephalitis/ West Nile encephalitis Zika fetal abnormalities, sexual transmission HIV infects CD4 T cells Retrovirus replication https://www.youtube.com/watch?v=odRyv7V8LAE Retrovirus replication https://www.youtube.com/watch?v=odRyv7V8LAE Reverse transcriptase for RNA quantification Retrovirus replication https://www.youtube.com/watch?v=odRyv7V8LAE Lentivirus-based gene delivery, or stable knock down Antiviral drugs target multiple stages of the HIV life cycle Human endogenous retroviruses •5-8% of the human genome, determined by homology to known retroviral sequences. •Not capable of replication due to containing many mutations, methylation, etc. •Can regulate nearby and distant genes, insert promoters, alternate splice sites •May modulate expression of protooncogenes Neighbor-joining tree of integrase protein sequences. Scientific Reports 5, Article number: 15644 (2015) Influenza replication Antigenic Shift vs. Antigenic Drift Replication of DNA virus Immune responses to viral pathogens The role of interferons in limiting viral infection Innate versus adaptive immune responses Flaviviral pathogens: Antigenically similar but induce varied clinical diseases Zika Virus 1930s 1938 2015 Japanese Encephalitis Virus Yellow Fever Virus Dengue Virus …but only neutralize the virus used to induce them 120 120 100 100 80 60 40 20 0 -11.3 100 Neutralization (%) Flavivirus cross-reactive antibodies are produced after challenge YFV PRNT Neutralization (%) Neutralization (%) JEV PRNT 80 60 40 20 -9.3 -7.3 -5.3 -3.3 1/Serum dilution (log10 ) DENV1 PRNT 0 -11.3 -9.3 -7.3 -5.3 -3.3 1/Serum dilution (log10 ) Saline Sera DENV1 Sera JEV Sera 80 YFV Sera 60 40 20 0 -11.3 -9.3 -7.3 -5.3 -3.3 1/Serum dilution (log10 ) Saron et al Science Advances 2018 Within the DENV serocomplex, antibodies and pre-existing REVIEWS immunity can contribute to pathology and disease Primary infection Secondary homologous infection CD8 + T cell TCR MHC class I MHC class II Serotype 1 TCR DENV antigenpresenting DC Selection of serotype 1-specific T and B cells CD4 + T cell Pre-existing antibodies Extracellular granule Infected FcR + cell Serotype 2 MC FcR Antibodydependent enhanced replication Excessive cytokine production by infected cells Antibody-dependent enhaced infection Serotype 1 Neutralizing antibodies Serotype Ispecific B cell Secondary heterologous infection Weakly-neutralizing antibodies promote virus opsonization Limited infection Intracellular granule Increased MC degranulation through FcR-dependent activation by virusimmune complexes Infected APC Increased killing of DENV-infected cells by NK cells recognizing antibodies bound to viral antigens NK cell Release of MC granules, cytokines, proteases, etc. Antibody-enhanced mast cell activation, leading to increased vascular permeability Release of cytotoxic granules, cytokines, etc. Antibody-dependent cellular cytotoxicity Fig.4 | Antibody-dependent pathologies during dengue virus infections. Pre-existing antibodies against dengue virus (DENV) interact with many different cell types, and there are several theories for how these can contribute to severe St. John & Rathore Nature Reviews Immunology 2019 Antibody-enhanced DENV severity is particularly well supported in infants born to DENV-immune mothers Emerging flavivirus, Zika, is closely related to dengue • • • Cross-reactive, non-neutralizing immunity is typical of flavivirus infection Dengue antibodies can enhance Zika infection (Dejnirattisai et al Nat Immunol 2016) Many Zika virus epidemic regions are endemic for dengue Does maternal dengue immunity influence the risk of fetal abnormalities? Animal models show dengue enhancement of Zika congenital defects DENV-immune mothers have antibodies that cross-react with ZIKV. Rathore & Saron et al Science Advances 2019 Incidence of microcephaly is significantly enhanced by dengue cross-reactive antibodies Rathore & Saron et al Science Advances 2019 Zika reduces cortical thickness, which is more severe with maternal dengue immunity Rathore & Saron et al Science Advances 2019 Nature Reviews Drug Discovery Zika-reduces cortical thickness, which is more severe with maternal dengue immunity submitted Maternal dengue-immunity promotes high titer fetal infection and incidence of infection ZIKV + ND Percentage PCR-postive 100 80 60 40 20 0 Maternal Naive Naive DENV2 4G2 Immunity Fetal mice ZIKV + + + What is the antibody dependent mechanism? Rathore & Saron et al Science Advances 2019 Enhanced infection or translocation? Downloaded from http://advances.s Rathore & Saron et al Science Advances 2019 Fetal Neonatal Fc Receptor (FcRN) promotes fetal infection Mother’s spleen Rathore & Saron et al Science Advances 2019 Placenta FcRN promotes uptake of ZIKV within trophoblasts and fetal endothelial cells Fetal endothelial cells Trophoblast cells Rathore & Saron et al Science Advances 2019 Rathore & Saron et al Science Advances 2019 p://advances.sciencemag.org/ on June 2 Therapeutic targeting of the FcRN receptor with a mAb improves microcephaly phenotype DENV antibodies promote translocation of ZIKV across human endothelial monolayers Downloaded from http://advances. Downloaded from http://advanc Rathore & Saron et al Science Advances 2019 Dengue immunity enhances ZIKV pathogenesis Recently, ZIKV-enhancing antibodies were confirmed to Maternal dengue immunity with microcephaly be associated • enhances the microcephaly phenotype in fetal mice • results increased viral titer in infected mice • increases the proportion of fetuses infected (100%) in humans Vertical transmission of ZIKV in DENV immune mothers is promoted by FcRN Serocomplex cross-reactive cellular responses are also induced Splenocyte Proliferation (% of control) 180 Saline DENV1 160 YFV * JEV 140 120 * * * * * * * * * 100 Control Ag DENV1 Ag YFV Ag JEV Ag DENV2 Ag Saron et al Science Advances 2018 Serocomplex cross-reactive immunity can prime for early protection during secondary infection ** 200 viral load (% of control) Control DENV1 YFV JEV 150 ns ns 100 * * * ns ns * 50 0 Serum Transfer Secondary Infection T cell Transfer Saron et al Science Advances 2018 Observed a unique activation of T effector memory cells WS Thy1.2+CD4+CD44+CD62−/loPD-1+CXCR5+BCL6+ Serocomplex crossreactive T effector memory cells become T follicular helper cells and promote germinal center responses T cell help Serotype 1 B cell 1 CD4 T cell Serotype 2 Primary Serocomplex 2 Challenge OR Naive T cell selection + CD8 T cell epitope T cell + DENV primary infection Naive T cell Memory formation s dary Thy1.2+ T cell transfer JEV, YFV, DENV 2 Anti-DENV memory T cells 5 Anti-serotype 1 neutralizing antibodies Cross-reactive CD4 epitope 10w Secondary Challenge (DENV) Flavivirus serocomplex 2 primary infection Cross-protection against serocomplex 2 T lymphocyte repertoire Memory recall and effective containment St. John & Rathore Nature Reviews Immunology 2019 Recall of cross-reactive CD4 + memory T cells Increased kinetics of serocomplex 2-specific neutralizing antibody production Saron et al Science Advances 2018 WS Serocomplex crossreactive T effector memory cells become T follicular helper cells and promote germinal center responses T cell help CD4 T cell DENVactivated DC Naive T cell Memory formation Anti-DENV memory T cells 5 Anti-serotype 1 neutralizing antibodies Cross-reactive CD4 epitope T lymphocyte repertoire Memory recall and effective containment St. John & Rathore Nature Reviews Immunology 2019 C ti ti d D Memory Plasma B cell B cell Capsule HEV B cell follicle CD8+ T cell CD4 T cell + TFH cell TCR MHC class II Re-entry of memory anti-DENV T FH cells into LNs during memory recall TCR MHC class I T cell zone Activated DC Efferent lymphatic Peripheral blood vessel DENV primary infection Memory TFH cell Subcapsular sinus Serotype 2 CD8 + T cell epitope T cell 2 Germinal centre Antigen presentation Serocomplex 2 in T cell zone T cell selection + s dary Movement of TFH cells between germinal centres Serotype 1 B cell 1 Afferent lymphatic Recall of cross-reactive CD4 + memory T cells Release of DENV-specific effector T cells from LNs Activated skin-homing effector memory CD8+ T cell TEMRA cell p c g A ta a re e is w w to in T to w n fo w p c ly ti fr a m y w v Fig. 3 | Adaptive T cell responses during dengue virus infection. Activated dendritic cells (DCs), some having been infected by dengue virus (DENV) in the skin, arrive in the Flavivirus draining lymph node (LN) through afferent lymphatics, where they present antigen to serocomplex 2+ bothinfection CD4 and CD8+ T cells for the initiation of the adaptive immune response. CD4+ primary T cells, activated by DENV antigen presented by DCs, have the potential to become T follicular helper (TFH) cells. These migrate from T cell zones to the periphery of the B cell Cross-protection follicle in the LN, where they subsequently participate in the germinal centre reaction against andserocomplex promote the 2 development of DENV-specific memory B cells and plasma cells. DENV-specific memory T cells acquire unique surface markers such as CD44 (in mice) and CD45RO (in humans)34. TFH cells are able to traffic to new follicles and to re-enter Increased kinetics of 34. During a secondary DENV infection, memory CD8+ T cells acquire a germinal centres serocomplex 2-specific E skin-homing phenotype (CXCR3+CCR5 +CL A+)62. These cells can exit the LN through neutralizing antibody production efferent lymphatics and re-enter circulation and theoretically have the potential to home to the skin for clearance of DENV. Similarly , CD4 + T cells can also acquire a cytotoxic in + phenotype (CD45RAhiCCR7 lowGPR56+CX 3CR1+granzyme ) and known as T effector2018 Saron et are al Science Advances WS Serocomplex crossreactive T effector memory cells become T follicular helper cells and promote germinal center responses T cell help CD4 T cell DENVactivated DC Naive T cell Memory formation Anti-DENV memory T cells 5 Anti-serotype 1 neutralizing antibodies Cross-reactive CD4 epitope T lymphocyte repertoire Memory recall and effective containment St. John & Rathore Nature Reviews Immunology 2019 C ti ti d D Memory Plasma B cell B cell Capsule HEV B cell follicle CD8+ T cell CD4 T cell + TFH cell TCR MHC class II Re-entry of memory anti-DENV T FH cells into LNs during memory recall TCR MHC class I T cell zone Activated DC Efferent lymphatic Peripheral blood vessel DENV primary infection Memory TFH cell Subcapsular sinus Serotype 2 CD8 + T cell epitope T cell 2 Germinal centre Antigen presentation Serocomplex 2 in T cell zone T cell selection + s dary Movement of TFH cells between germinal centres Serotype 1 B cell 1 Afferent lymphatic Recall of cross-reactive CD4 + memory T cells Release of DENV-specific effector T cells from LNs Activated skin-homing effector memory CD8+ T cell TEMRA cell p c g A ta a re e is w w to in T to w n fo w p c ly ti fr a m y w v Fig. 3 | Adaptive T cell responses during dengue virus infection. Activated dendritic cells (DCs), some having been infected by dengue virus (DENV) in the skin, arrive in the Flavivirus draining lymph node (LN) through afferent lymphatics, where they present antigen to serocomplex 2+ bothinfection CD4 and CD8+ T cells for the initiation of the adaptive immune response. CD4+ primary T cells, activated by DENV antigen presented by DCs, have the potential to become T follicular helper (TFH) cells. These migrate from T cell zones to the periphery of the B cell Cross-protection follicle in the LN, where they subsequently participate in the germinal centre reaction against andserocomplex promote the 2 development of DENV-specific memory B cells and plasma cells. DENV-specific memory T cells acquire unique surface markers such as CD44 (in mice) and CD45RO (in humans)34. TFH cells are able to traffic to new follicles and to re-enter Increased kinetics of 34. During a secondary DENV infection, memory CD8+ T cells acquire a germinal centres serocomplex 2-specific E skin-homing phenotype (CXCR3+CCR5 +CL A+)62. These cells can exit the LN through neutralizing antibody production efferent lymphatics and re-enter circulation and theoretically have the potential to home to the skin for clearance of DENV. Similarly , CD4 + T cells can also acquire a cytotoxic in + phenotype (CD45RAhiCCR7 lowGPR56+CX 3CR1+granzyme ) and known as T effector2018 Saron et are al Science Advances Immune responses can be protective, but viruses evade immunity and it can drive virus evolution Immune responses can influence functional disease outcomes, including viral replication, cellular tropism, and immune mediated pathologies