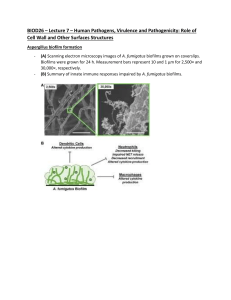
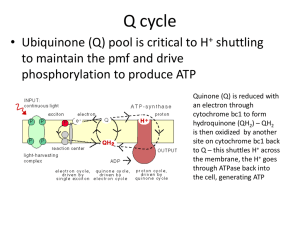
BIOFILM IN ENDODONTICS DR. LAXMIPRIYA C H POSTGRADUATE STUDENT DEPARTMENT OF CONSERVATIVE DENTISTRY CONTENTS • Introduction • Definition of biofilm • Criteria for biofilm • Composition of biofilm • Characteristics of biofilm • Development of biofilm • Stages of biofilm formation • Resistance of microbes in biofilm to antimicrobials • Endodontic biofilms • Types of Endodontic biofilms -intracanal biofilm -Extraradicular biofim -Periapical biofilm -Biomaterial centered infection • Methods to study biofilm • Current and future therapeutic strategies against endodontic biofilm • Summary • Conclusion • References INTRODUCTION APICAL PERIODONTITIS - biofilm-mediated infection Biofilm - protects bacteria from host defenses - increase their resistance to intracanal disinfecting protocols Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) Understanding the virulence of these endodontic microbiota within biofilm essential for the development of novel therapeutic procedures for intracanal disinfection Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) Disruption of biofilms Killing of bacteria in biofilm Necessary to effectively treat APICAL PERIODONTITIS Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) Definition Biofilm can be defined as a sessile multicellular microbial community characterized by cells that are firmly attached to a surface and enmeshed in a self-produced matrix of extracellular polymeric substance (EPS), usually a polysaccharide Cohen 11th edition Biofilm is a mode of microbial growth where dynamic communities of interacting sessile cells are irreversibly attached to a solid substratum, as well as each other, and are embedded in a self-made matrix of extracellular polymeric substances (EPS) Ingle’s 6th edition A microbial biofilm is considered a community that meets the following four basic criteria – caldwell et al The microorganisms living in the community must possess the abilities to self-organize - autopoiesis resist environmental perturbations - homeostasis Jhajharia, et al. Biofilm in endodontics: A review Journal of International Society of Preventive and Community Dentistry January-February 2015, Vol. 5, No. 1 must be more effective in association than in isolation synergy respond to environmental changes as a unit rather than single individuals - communality Jhajharia, et al. Biofilm in endodontics: A review Journal of International Society of Preventive and Community Dentistry January-February 2015, Vol. 5, No. 1 COMPOSITION OF BIOFILM A fully developed biofilm - heterogeneous arrangement of microbial cells on a solid surface Biofilm consists of Matrix material 85% volume 15% cells T. Akshay Satwik, Pradeep Solite, R. Sarah Sathiyawathie. Role of biofilms in endodontics. Drug Invention Today .Vol 11 (4) 2019 Surface adherent surface cells -main component of the biofilm Water channels - primitive circulatory system T. Akshay Satwik, Pradeep Solite, R. Sarah Sathiyawathie. Role of biofilms in endodontics. Drug Invention Today .Vol 11 (4) 2019 Typically, a viable, fully hydrated biofilm appears as ‘‘tower’’ or ‘‘mushroom’’shaped structures adherent to substrate The overall shape of a biofilm structure is determined by the shear forces generated by the flushing of fluid media. Ingle’s Endodontics 6th edition As biofilm get matured - structure and composition -modified according to the environmental conditions Biofilm mediated mineralization occurs when the metal ions including Ca2+, Mg2+, and Fe3+ readily bind and precipitate within an ionic biofilm under a favorable environment T. Akshay Satwik, Pradeep Solite, R. Sarah Sathiyawathie. Role of biofilms in endodontics. Drug Invention Today .Vol 11 (4) 2019 Schematic representation of the structure of a mature biofilm Ingle’s Endodontics 6th edition CHARACTERISTICS OF BIOFILM Bacteria in a biofilm state Distinct capacity to survive tough growth and environmental conditions Ingle’s Endodontics 6th edition This unique capacity of bacteria in a biofilm state is due to the following features: biofilm structure protects the residing bacteria from environmental threats structure of biofilm permits trapping of nutrients and metabolic cooperativity between resident cells of same species and/or different species; Ingle’s Endodontics 6th edition biofilm -display organized internal compartmentalization, which allows bacterial species with different growth requirements to survive in each compartment Bacterial cells in a biofilm community may communicate and exchange genetic materials to acquire new traits. Ingle’s Endodontics 6th edition PROTECTION OF BIOFILM BACTERIA FROM ENVIRONMENTAL THREATS Bacteria are capable of producing polysaccharides, either as cell surface structures (capsule) or as extracellular excretions (EPS) EPS covers biofilm communities and creates a microniche favorable for the long-term survival and functioning of the bacterial communities Ingle’s Endodontics 6th edition NUTRIENT TRAPPING AND ESTABLISHMENT OF METABOLIC COOPERATIVITY IN A BIOFILM Highly permeable and interconnected water channelsmaterial exchange. water channel connects the outer fluid medium with the interior of the biofilm ensure nutrient availability to microbial communities deep inside the biofilm structure Ingle’s Endodontics 6th edition ORGANIZED INTERNAL COMPARTMENTALIZATION IN BIOFILM A mature biofilm- displays gradients in the distribution of nutrients, pH, oxygen, metabolic products, and signaling molecules within the biofilm create different microniche that can accommodate diverse bacterial species within a biofilm. Ingle’s Endodontics 6th edition Schematic diagram representing cell–cell communication in a biofilm. Some bacteria can produce chemical signals (green) and other bacteria from the same species or from different species or strain can respond to them (red) Ingle’s Endodontics 6th edition BACTERIAL CELLS RESIDING IN A BIOFILM COMMUNICATE, EXCHANGE GENETIC MATERIALS, AND ACQUIRE NEW TRAITS Communications between bacterial cells residing in a biofilm is attained through signaling molecules Process called QUORUM SENSING Ingle’s Endodontics 6th edition Schematic diagram showing quorum sensing in biofilm bacteria Ingle’s Endodontics 6th edition DEVELOPMENT OF BIOFILM 3 major components involved in biofilm formation Bacterial cells solid surface Fluid medium Ingle’s Endodontics 6th edition Ingle’s Endodontics 6th edition Stages of biofilm formation Gunnel svensater & gunnar bergenholtz. Biofilms in endodontic infections. Endodontic topics 2004, 9, 27–36 Ingle’s Endodontics 6th edition Schematic diagram showing co-aggregation and co-adhesion between different bacterial cells forming biofilm. Ingle’s Endodontics 6th edition Detachment – important role in shaping the morphological characteristics It is also an “active dispersal mechanism” or “seeding dispersal” detached cells form resistance traits source of persistent infections T. Akshay Satwik, Pradeep Solite, R. Sarah Sathiyawathie. Role of biofilms in endodontics. Drug Invention Today .Vol 11 (4) 2019 Erosion Sloughing continual detachment of single cell and small portions of biofilm a process of rapid, massive loss of biofilm T. Akshay Satwik, Pradeep Solite, R. Sarah Sathiyawathie. Role of biofilms in endodontics. Drug Invention Today .Vol 11 (4) 2019 Resistance of Microbes in Biofilm to Antimicrobials The mechanisms responsible include: resistance associated with the extracellular polymeric matrix resistance associated with growth rate and nutrient availability resistance associated with the adoption of resistance phenotype Ingle’s Endodontics 6th edition Schematic diagram showing factors contributing to resistance against antimicrobial agents. Ingle’s Endodontics 6th edition Endodontic biofilms Endodontic microbiota -less diverse -oral microbiota. Progression of infection alters the nutritional and environmental status -root canal more anaerobic with depleted nutritional levels. Lakshmi Narayanan and Vaishnavi: Endodontic microbiology . Journal of Conservative Dentistry OctDec 2010. Vol 13 ,Issue 4 Endodontic infection Primary Secondary PRIMARY ENDODONTIC INFECTIONS - polymicrobial They are predominantly Bacteroides, Prophyromonas, Prevotella, Fusobacterium, Treponema, Peptostreptococcos, Eubacterium & Camphylobacter species. Prasanna Neelakantan ,Monica Romero , Jorge Vera , Umer Daood , Asad U. Khan , Aixin Yan ,Gary Shun Pan Cheung . Biofilms in Endodontics—Current Status and Future Directions. Int. J. Mol. Sci. 2017 SECONDARY INFECTIONS - microbial flora - able to survive harsh conditions Studies have shown the prevalence of certain species in teeth with post-treatment infection Enterococci, Streptococci, Lactobacilli, Actinomyces & fungi In particular, a high proportion of Enterococcus fecalis in cases with persistent apical periodontitis Prasanna Neelakantan ,Monica Romero , Jorge Vera , Umer Daood , Asad U. Khan , Aixin Yan ,Gary Shun Pan Cheung . Biofilms in Endodontics—Current Status and Future Directions. Int. J. Mol. Sci. 2017 Complete disinfection of root canal is very difficult to achieve because of persistent microbes in anatomical complexities and apical portion of root canal Biofilm - survives unfavorable environmental and nutritional condition - the root canal environment will favor biofilm formation Lakshmi Narayanan and Vaishnavi: Endodontic microbiology . Journal of Conservative Dentistry OctDec 2010. Vol 13 ,Issue 4 Biofilm mode of bacterial growth offers other advantages such as resistance to antimicrobial agents increase in the local concentration of nutrients opportunity for genetic material exchange Ingle’s Endodontics 6th edition ability to communicate between bacterial populations of same and/or different species produce growth factors across species boundaries. Ingle’s Endodontics 6th edition Endodontic bacterial biofilms can be categorized as Intracanal biofilms Extraradicular biofilms Periapical biofilms Biomaterial-centered infections Ingle’s Endodontics 6th edition Intracanal biofilms Intracanal microbial biofilms are microbial biofilms formed on the root canal dentine of an endodontically infected tooth A detailed description on the intracanal bacterial biofilm was documented by Nair in 1987 Ingle’s Endodontics 6th edition Nair reported that despite instrumentation, irrigation, and obturation for single-visit treatment of mandibular first molars with primary apical periodontitis, microorganisms persisted within biofilms in untouched areas of canals and isthmuses intracanal biofilms. Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) Intracanal biofilm Intracanal microbiota in an endodontically infected teeth existed as both loose collection and biofilm structures, made up of cocci, rods, and filamentous bacteria Monolayer and/or multilayered bacterial biofilms were found to adhere to the dentinal wall of the root canal The extracellular matrix material of bacterial origin -interspersed with the cell aggregates in the biofilm. Ingle’s Endodontics 6th edition Morphologically distinct types of bacteria - observed in these biofilms Bacterial microcolonies - coaggregation of single morphological type and/or several morphological types of bacteria In a multispecies biofilm - proportion and number of different bacterial species varied according to the stage of maturation Ingle’s Endodontics 6th edition Intracanal biofilms displayed characteristic bacteria– dentine wall relationship and distinct patterns in the organization of microbes in the biofilm Studies have established the ability of E.faecalis to resist starvation and develop biofilms under different environmental and nutrient conditions Ingle’s Endodontics 6th edition Sen et al investigated the root canal walls of infected teeth by SEM Bacteria formed dense colonies on the canal walls as well as in intra- and inter-tubular dentin In addition to bacteria, fungi were capable of forming dense, but separate colonies all over the root canal walls Mohammadi Z, Palazzi F, Giardino L , Shalavi S.Microbial Biofilms in Endodontic Infections: An Update Review Biomed J 2013;36:59-70 George et al - evaluated the ability of E. faecalis to develop biofilm Aerobic Anaerobic Nutrient-rich Nutrient-deprive d conditions Mohammadi Z, Palazzi F, Giardino L , Shalavi S.Microbial Biofilms in Endodontic Infections: An Update Review Biomed J 2013;36:59-70 Stage 1 E. faecalis cells adhered and formed microcolonies on the root canal dentine surface Stage 2 They induced bacterial-mediated dissolution of the mineral fraction from the dentine substrate. Stage 3 This localized increase in the calcium and phosphate ions will promote mineralization or calcification of the E. faecalis biofilm Ingle’s Endodontics 6th edition The mature biofilm structure formed after 6 weeks of incubation showed signs of mineralization Obvious signs of dentine surface degradation under nutrient-deprived environment Ingle’s Endodontics 6th edition Degradation of dentine substrate - consequence of the interaction of bacteria and their metabolic products on dentine Inherent capacity- E. faecalis - may contribute to its persistence in endodontically treated teeth. Ingle’s Endodontics 6th edition Scanning electron microscopy images showing the morphology of Enterococcus faecalis biofilms formed on root canal dentine under A, nutrient-deprived condition after 1 week, B, nutrient-deprived condition after 4 weeks, C, nutrient-rich condition after 1 week, and D, nutrient-rich condition after 4 weeks. Ingle’s Endodontics 6th edition Investigations -biting force-induced retrograde fluid movement into the apical portion of the root canal Cyclic influx of ion-rich tissue fluid into the apical portion of the root canal can promote persistence of bacteria as biofilms and their mineralization while internal resorption can be a consequence of bacterialmediated substrate dissolution. Ingle’s Endodontics 6th edition EXTRARADICULAR MICROBIAL BIOFILMS Extraradicular microbial biofilms root surface biofilms are microbial biofilms formed on the root (cementum) surface adjacent to the root apex of endodontically infected teeth Extraradicular biofilms Asymptomatic Periapical periodontitis chronic apical abscesses associated with sinus tracts. Ingle’s Endodontics 6th edition Tronstad et al - examined 10 root tips removed during surgical treatment of root-filled teeth with post-treatment disease Mature bacterial biofilms were found in many areas of the apical root surfaces in all clinical specimens They observed bacterial biofilms in the areas of the root surfaces between fibers and cells and in crypts and holes Mohammadi Z, Palazzi F, Giardino L , Shalavi S.Microbial Biofilms in Endodontic Infections: An Update Review Biomed J 2013;36:59-70 The biofilm contained varying degrees of extracellular matrix materials (glycocalyx) The root surface biofilms were mostly multispecies in nature Mohammadi Z, Palazzi F, Giardino L , Shalavi S.Microbial Biofilms in Endodontic Infections: An Update Review Biomed J 2013;36:59-70 The extraradicular biofilm structures were dominated by cocci and short rods, with cocci attached to the tooth substrate Filamentous and fibrillar forms were also observed in the biofilm Mohammadi Z, Palazzi F, Giardino L , Shalavi S.Microbial Biofilms in Endodontic Infections: An Update Review Biomed J 2013;36:59-70 A smooth, structureless biofilm -extracellular matrix material with embedded bacterial cells was noticed to coat the apex of the root tip adjacent to the apical foramen Ricucci et al - presence of calculus-like deposit on the root apex of teeth extracted due to post-treatment periapical periodontitis. Ingle’s Endodontics 6th edition Harn et al - calculus-like deposits on apical root surface of tooth presented with lesion refractory to conventional root canal treatment These calcified biofilms were associated with periapical inflammation and delayed periapical healing in spite of adequate orthograde root canal treatment Ingle’s Endodontics 6th edition Extraradicular microbial biofilm formed on the root surface. It is A, multispecies or B, smooth and structureless in nature Ingle’s Endodontics 6th edition PERIAPICAL MICROBIAL BIOFILMS Periapical microbial biofilms are isolated biofilms found in the periapical region of an endodonticallyinfected teeth. Periapical biofilms may or may not be dependent on the root canal. Ingle’s Endodontics 6th edition The microbiota in the majority of teeth associated with apical periodontitis restricted to the root canal most of the microbial species that infect the root canal are opportunistic pathogens do not have the ability to survive host defense mechanism in the periapical tissues Ingle’s Endodontics 6th edition Actinomyces P.propionicum Asymptomatic periapical lesions refractory to endodontic treatment Ability to overcome host defense mechanisms, thrive in the inflamed periapical tissue - induce a periapical infection Ingle’s Endodontics 6th edition Actinomyces species - grow in microscopic or macroscopic aggregates -3 to 4mm Sulfur granules - yellow granular appearance. Ray fungus Aggregation of cells -biofilm - that differentiate, communicate, cooperate, and deploy collective defense against biological antimicrobials Ingle’s Endodontics 6th edition Periapical region - ‘‘patrolled’’ by PMNs & macrophages-phagocytose incoming planktonic bacteria easily Unable to engulf bacteria in matrix-enclosed biofilm structure Ingle’s Endodontics 6th edition Sunde et al - reported the incidence of ‘‘sulfur granules’’ in nine refractory periapical lesions and found bacteria in seven. A. israelii, A. viscosus, A. naeslundii, Actinomyces meyeri were identified in five granules Ingle’s Endodontics 6th edition Many of the ‘‘sulfur granules’’ were calcified Source for mineralization - the inflammatory exudate and/or the activity of the periapical bacteria Lysis of adherent bacterial cells in a biofilm would induce cell calcification Mohammadi Z, Palazzi F, Giardino L , Shalavi S.Microbial Biofilms in Endodontic Infections: An Update Review Biomed J 2013;36:59-70 BIOMATERIAL-CENTERED INFECTION Biomaterial-centred infection (BCI) is caused when bacteria adheres to an artificial biomaterial surface and forms biofilm structures Ingle’s Endodontics 6th edition Presence of biomaterials in close proximity to the host immune system can increase the susceptibility to BCI BCI is one of the major complications associated with prosthesis and/or implant-related infections Ingle’s Endodontics 6th edition Because biofilms - extremely resistant to host defense mechanisms and antibiotic treatments BCI are rarely resolved - often the only solution to an infected biomaterial such as Implant is its surgical removal Ingle’s Endodontics 6th edition BCI usually reveals opportunistic invasion by nosocomial Organisms Staphylococcus, S. aureus, enterococci, streptococci, P. aeruginosa, and fungi - commonly isolated from infected biomaterial surfaces Mohammadi Z, Palazzi F, Giardino L , Shalavi S.Microbial Biofilms in Endodontic Infections: An Update Review Biomed J 2013;36:59-70 Bacterial adherence to a biomaterial surface - three phases phase 1 transport of bacteria to biomaterial surface phase 2 initial, non-specific adhesion phase Phase 3 specific adhesion phase. Ingle’s Endodontics 6th edition Bacterial strains that do not produce EPS Less adherent & less pathogenic Rapidly destroyed by the immune system. These features highlight the need to prevent bacterial adherence and biofilm formation to prevent BCI Ingle’s Endodontics 6th edition In endodontics - biomaterial-centered biofilms would form on root canal obturating materials Intraradicular Extraradicular Obturating material is within the root canal space or has it extruded beyond the root apex Ingle’s Endodontics 6th edition A study investigated the initial biofilm-forming ability of root canal isolates such as E. faecalis, S. sanguinis, S. intermedius,S. pyogenes, S. aureus, F. nucleatum, P. acnes, P. gingivalis, and P. intermedia on gutta-percha points in vitro Ingle’s Endodontics 6th edition E. faecalis & S. sanguinis biofilms - significantly thicker than those of S. intermedius, S. pyogenes, and S. aureus F. nucleatum, P. acnes, P. gingivalis, and P. intermedia did not form biofilms on gutta-percha The findings suggested that Gram-positive facultative anaerobes have the ability to colonize and form extracellular matrices on gutta-percha points Ingle’s Endodontics 6th edition Scanning electron microscopy image of A, microbial biofilm formed on extruded gutta-percha point from a clinical sample and B, E. faecalis biofilm formed on gutta-percha in vitro. Ingle’s Endodontics 6th edition METHODS TO STUDY BIOFILM Atomic force microscopy (afm) Nuclear magnetic resonance (NMR) Green fluorescent protein (GFP) tagging Fourier transform infrared (FTIR) spectroscopy SEM & TEM FISH confocal laser scanning microscopy (CLSM) flow cytometry Jhajharia, et al. Biofilm in endodontics: A review Journal of International Society of Preventive and Community Dentistry January-February 2015, Vol. 5, No. 1 CURRENT AND FUTURE THERAPEUTIC STRATEGIES AGAINST ENDODONTIC BIOFILM Irrigants for biofilm eradication Intracanal medicament for biofilm eradication Laser-assisted eradication of biofilms Effect of Lactobacillus plantarum LTA Effect of nanoparticles, photodynamic therapy, ozone, and enzymes Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) Irrigants for biofilm eradication Sodium hypochlorite (NaOCl) E. FECALIS lipoteichoic acid (LTA) with NaOCl Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) Chlorhexidine (CHX) digluconate CHX cannot be used as main root canal irrigant because it does not have tissue solvent activity Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) EDTA In addition to smear layer removal, EDTA irrigation can be beneficial in disruption of biofilm Ozdemir et al - demonstrated that combination of EDTA and NaOCl significantly reduced the amount of intracanal biofilm in both young and old aged biofilms Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) Soares et al reported that the NaOCl-EDTA alternating irrigation was a promising regimen for elimination of intracanal E. faecalis biofilms Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) Irrigant Activation Mechanisms Complexity of the root canal anatomy and tenacious nature of the biofilms dictate that simple delivery of antimicrobial agents is not sufficient for disinfection of root canal systems. Irrigation activation -satisfactorily deliver these antimicrobial agents into the complex anatomy, interfere with the adhesive mechanisms by inducing shear stress and disrupt the biofilms Prasanna Neelakantan ,Monica Romero , Jorge Vera , Umer Daood , Asad U. Khan , Aixin Yan ,Gary Shun Pan Cheung . Biofilms in Endodontics—Current Status and Future Directions. Int. J. Mol. Sci. 2017 RINSENDO GENTLE WAVE ENDOVAC PIPS ENDOACTIVATOR VATEA SYSTEM Intracanal medicament for biofilm eradication Calcium hydroxide Release of aqueous hydroxyl ions to raise pH so that microbes cannot survive Elevated pH alters membrane integrity Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) Damage in the lipid moieties of bacterial virulence factors - unique detoxification mechanism of CH E. faecalis - resistant to CH- proton pump for and inhibitory dentin buffering effect CH can also inactivate lipopolysaccharide (LPS) in gram negative bacteria, via hydrolysis of fatty acid in the lipid moiety Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) Chlorhexidine Positively charged CHX molecules interact with negatively charged membrane phospholipids CHX - superior antifungal activity compared to CH, up to 400 μm depth dentinal tubules Provides substantive antimicrobial activity Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) Human beta defensins Cationic antimicrobial peptides that are critical host defense against microbes They bind to the negatively charged molecules on bacterial surface and disrupt bacterial membranes HBD-3 is strongly inhibitory, whereas HBD-1, -2, and -4 have weak antimicrobial effects on E. faecalis Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) HBD-1, -2, -3, and -4 are produced in normal and inflamed dental pulp They may protect the pulp from inflammation induced by LTA of gram-positive bacteria and LPS of gram-negative bacteria Synthetic HBD-3 consisting of the C terminal 15 amino acids (HBD3-C15) was reported to be effective for disinfecting endodontic biofilm including C. albicans Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) Triple antibiotic paste Triple antibiotic paste (TAP) - a mixture of metronidazole, ciprofloxacin & minocycline It is effective on infected dentin, intracanal biofilms, and the majority of endodontic pathogens But its toxicity to residual undifferentiated cells and periapical tissues limits its application in REP Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) Laser-assisted eradication of biofilms A low power laser directed at the access cavity combined with a photosensitizing agent was bactericidal on S.intermedius biofilms in root canals, but less effective than NaOCl (3%) irrigation Er:YAG laser was effective on apical root apex biofilms in vitro However, endodontic pathogens in biofilms were difficult to eradicate despite direct laser exposure ex vivo Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) Effect of Lactobacillus plantarum LTA L. plantarum - probiotic - antiinflammatory & anti-biofilm effect LTA inhibit biofilm formation by : controlling gene expression Quorum sensing inhibiting EPS production Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) LTA also disrupted preformed biofilm of E. faecalis and S. aureus L. plantarum LTA reduced not only mono-species biofilm, but also multispecies biofilm consisting of A. naeslundii,E. faecalis, Lactobacillus salivarius, and S. mutans Cooperatively enhanced disruption of oral multispecies biofilm when combined with CH and CHX intracanal medicaments Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) Effect of nanoparticles, photodynamic therapy, ozone, and enzymes Nanoparticles Synthesized from powders of silver, copper oxide, and zinc oxide broad antimicrobial applications Generate reactive oxygen species (ROS) -cytotoxic for bacteria Higher surface area - greater potential for bacterial interactions Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) Numerous positively charged nanoparticles accumulate on negatively charged bacterial cell membranes, which increase permeability to destroy cells Additionally, cationic nanoparticles adhere to negatively charged dentin surface to prevent biofilm formation Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) Photodynamic therapy (PDT) PDT- photosensitizer is preferentially localized in tissue activated by appropriate wavelength light to generate reactive oxygen that kill bacteria Penetration of the activating light & photosensitizer may be limited within root canal structures. Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) When microorganisms were sensitized with methylene blue all bacterial species except E. faecalis (53% killing) were destroyed When this was followed by the addition of red light with an optical fiber, almost all (97%) E. faecalis biofilm bacteria in root canals were eliminated Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) Ozone Ozone gas (HealOzone, KaVo, Biberach, Germany) has yielded inconsistent result in destroying endodontic pathogens These inconsistencies may have been due to variation in concentration and duration of application There is conflicting evidence on its antimicrobial efficacy and reduced effects on sessile versus planktonic bacteria Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) Enzymes Natural plant extracts such as polyphenols, Morinda citrifolia, and turmeric Enzymes - dispersin B and proteinase K, have been proposed for treating biofilm medicated infections. But studies are needed to demonstrate their efficacy. Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) SUMMARY • Definition • Composition of biofilm • Characteristics of biofilm • Development of biofilm • Stages of biofilm formation • Resistance of microbes in biofilm to antimicrobials Endodontic biofilms Types of Endodontic biofilms -intracanal biofilm -Extraradicular biofim -Periapical biofilm -Biomaterial centered infection Various methods to study biofilms Therapeutic strategies against endodontic biofilm CONCLUSION Application of the biofilm concept to endodontics will play a vital role in helping us to understand, not only the pathogenic potential of the root canal microbiota but also the basis for new approaches for disinfection. Microorganisms adaptation under disease conditions and their organization in root canals are important issues - to obtain a clear understanding of how the root canal bacteria resist endodontic treatment measures Recent developments in biocompatible intracanal medicaments including synthetic HBDs and L. plantarum LTA could open up new avenues as an ideal therapeutic agent to eradicate endodontic biofilm. REFERENCES • Ingle’s Endodontics 6th edition • Cohen’s pathways of pulp 11 th edition • Yoo,Yeon-Jee et al. Endodontic biofilms: contemporary and future treatment options. Restorative Dentistry & Endodontics(2019), 44 (1) • Mohammadi Z, Palazzi F, Giardino L , Shalavi S.Microbial Biofilms in Endodontic Infections: An Update Review Biomed J 2013;36:59-70 • Gunnel svensater & gunnar bergenholtz. Biofilms in endodontic infections. Endodontic topics 2004, 9, 27–36 • T. Akshay Satwik, Pradeep Solite, R. Sarah Sathiyawathie. Role of biofilms in endodontics. Drug Invention Today .Vol 11 (4) 2019 • Lakshmi Narayanan and Vaishnavi: Endodontic microbiology . Journal of Conservative Dentistry Oct-Dec 2010. Vol 13 ,Issue 4 • Prasanna Neelakantan ,Monica Romero , Jorge Vera , Umer Daood , Asad U. Khan , Aixin Yan ,Gary Shun Pan Cheung . Biofilms in Endodontics—Current Status and Future Directions. Int. J. Mol. Sci. 2017 • Shwetha H B , Subhashini N, Chandra VR, Damodaran T, Anoop P S. Biofim in endodontics: A Review. IJOCR 2015; VOLUME 3(3) • Swimberghe RCD, Coenye T, De Moor RJG, Meire MA. Biofilm model systems for root canal disinfection: a literature review. International Endodontic Journal, 52, 604– 628, 2019 • Ramirez-Mora T, Retana-Lobo C, ValleBourrouet G .Biochemical characterization of extracellular polymeric substances from endodontic biofilms. PLoS ONE . 2018 13(11) • Jhajharia, et al. Biofilm in endodontics: A review Journal of International Society of Preventive and Community Dentistry January-February 2015, Vol. 5, No. 1