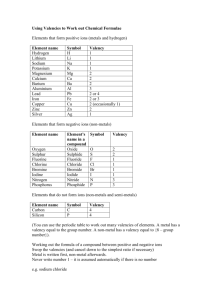
Prep Worksheet -1 Language of chemistry Q1. Answer the following questions:I. What is meant by the term symbol? Give two examples Ans- II. Explain the term valency. State why the valency of potassium metal is +1 and non metal chlorine is-1. Ans – III. What is meant by the term variable valency? give examples Ans – IV. Give the valency in each case and name the element:a) K b) Cr2O7 c) Cl d) Ni e) Br f) Ba g) I h) Zn i) ClO j) Al k) OH l) C m) CO n) N o) H p) Ca q) Li r) Mg s) Cr V. What do the symbols given below represent in a chemical equation:a) b) (s) c) (l) d) (g) e) (aq) VI. Explain the meaning of the term compound with a suitable example. State the main characteristics of a compound. Ans – VII. Explain the term chemical formula. State why the molecular formula of zinc carbonate is ZnCO3. Ans – VIII. Fill in the blanks :a) A symbol represent a short form of a /an…………………………[atoms/element/molecule] b) Compounds are also ……………………………..[heterogeneous/homogeneous]in nature. c) Variable valency is exhibited ,since electrons are lost from an element from the ………………………………….[valency/penultimate]shell. d) A chemical equation is a short hand form for a ……………………………….. [physical/chemical]change. IX. X. Balance the following simple equations:a) C + O2 O2 CO b) N2 + c) ZnS + O2 ZnO d) Al + O2 Al2O3 e) Mg + N2 Mg3N2 f) NO + O2 NO2 g) NaHCO3 + H2SO4 Na2SO4 H2O H2O h) NaOH + H2SO4 Na2SO4 i) FeCl3 + NaOH NaCl + j) CuSO4 + NaOH Na2SO4 Fe(OH)3 Write balanced equation for the following word equation:a) Potassium nitrate potassium nitrate + oxygen b) Calcium + water calcium hydroxide + hydrogen c) Iron + hydrochloric acid iron [II]chloride + hydrogen d) Nitrogen dioxide + water +oxygen nitric acid e) Lead[IV]oxide Lead monoxide + oxygen. NO + Cu(OH)2 +SO2 + CO2