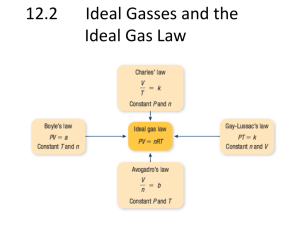
Name: ___________________________ Gas Laws Assignment 1. Convert the following temperatures from degrees Celsius to absolute temperature. [2] a. Dry ice sublimes into a gas at -78°C b. The hottest temperature recorded in Canada is 45°C 2. A container holds three gases: oxygen, carbon dioxide, and helium. The partial pressures of the oxygen and carbon dioxide are 104 kPa and 242 kPa, respectively. If the total pressure inside the container is 545 kPa, what is the partial pressure of the helium gas? [2] 3. A sample of methane gas occupies an initial volume of 5.25 L at an initial temperature of 200.0 K. The gas is heated to 300.0 K while the pressure and the amount of gas remain constant. Determine the new volume. [2] 4. A 1.75 L sample of ammonia gas increases in volume to 6.50 L when the pressure reaches 2.84 kPa. What was the original pressure of this gas? [2] Name: ___________________________ 5. A 450 mL sample of propane gas at 253 kPa and 15°C was compressed to 310 mL at a pressure of 405 kPa. Calculate the final temperature in Celsius. [3] 6. A balloon that contains 4.80 g of carbon dioxide gas has a volume of 12.0 L. Assume that the pressure and temperature of the balloon remain constant. What is the new volume of the balloon if an additional 0.50 mol of CO2 is added? [3] 7. A neon gas in a sign has a volume of 42.5 L at 25°C and 3.5 kPa. Calculate the mass of neon. Name: ___________________________ Gas Laws Assignment 8. Convert the following temperatures from degrees Celsius to absolute temperature. a. Dry ice sublimes into a gas at -78°C b. The hottest temperature recorded in Canada is 45°C 9. A container holds three gases: oxygen, carbon dioxide, and helium. The partial pressures of the oxygen and carbon dioxide are 104 kPa and 242 kPa, respectively. If the total pressure inside the container is 545 kPa, what is the partial pressure of the helium gas? (199kPa) 10. A sample of methane gas occupies an initial volume of 5.25 L at an initial temperature of 200.0 K. The gas is heated to 300.0 K while the pressure and the amount of gas remain constant. Determine the new volume. (7.88 L) 11. A 1.75 L sample of ammonia gas increases in volume to 6.50 L when the pressure reaches 2.84 kPa. What was the original pressure of this gas? (10.5 kPa) Name: ___________________________ 12. A 450 mL smaple of propane gas at 253 kPa and 15°C was compressed to 310 mL at a pressure of 405 kPa. Calculate the final temperature in Celsius. (45) 13. A balloon that contains 4.80 g of carbon dioxide gas has a volume of 12.0 L. Assume that the pressure and temperature of the balloon remain constant. What is the new volume of the balloon if an additional 0.50 mol of CO2 is added? (67 L) 14. A neon gas in a sign has a volume of 42.5 L at 25°C and 3.5 kPa. Calculate the mass of neon. (1.2 g)