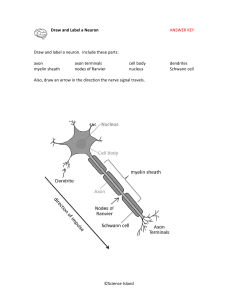
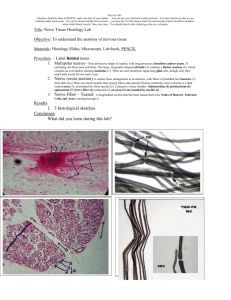
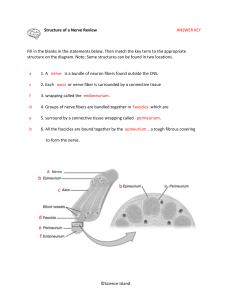
NERVE FIBERS. NERVE ENDINGS Using lectures (on the web-page of the department posted the presentation text and lectures), books, additional literature and other sources, students must to prepare the following theoretical questions: 1. General features and classification of nerve fibres. 2. Myelinated nerve fibers micro- and ultrastructure. 3. Mechanism of the myelinated nerve fibers formation. 4. Non-myelinated nerve fibers histophysiology. 5. Regeneration and degeneration of nerve fibers. 6. Nervous endings general morphofunctional characteristic. 7. Receptors classification and structure. 8. Effectors classification and structure. 9. Synapse. Interneuronal synapses classification and structure. 10. Symple reflectory ark compounds. Synapses functions. 11. Neuronal theory. Nervous system is the most complex system in the human body and is formed by a network of more than 100 million nerve cells (neurons), assisted by much numerous glial cells. Each neuron has a thousand interconnections with other neurons, forming a very complex system for communication. Neurons provide rapid communication between groups of serially disposed cells, permitting rapid transmission of information over long distances. NERVE FIBERS Nerve fibers consist of axons enveloped by a special sheath derived from cells of ectodermal origin. Groups of nerve fibers constitute the tracts of the brain, spinal cord, and peripheral nerves. Nerve fibers exhibit differences in their enveloping sheaths, related to whether the fibers are part of the central or the peripheral nervous system. Most axons in adult nerve tissue are covered by single or multiple folds of a sheath cell. In peripheral nerve fibers, the sheath cell is the Schwann cell, and in central nerve fibers it is the oligodendrocyte. Axons of small diameter are usually unmyelinated nerve fibers. MYELINATED FIBERS In myelinated fibers of the peripheral nervous system, the plasmalemma of the covering Schwann cell winds and wraps around the axon. The layers of membranes of the sheath cell unite and form myelin, a lipoprotein complex whose lipid component can be partly removed by standard histologic procedures. Myelin consists of many layers of modified cell membranes. These membranes have a higher proportion of lipids than do other cell membranes. Central nervous system myelin contains two major proteins: myelin basic protein and proteolipid protein. Several human demyelinating diseases are due to an insufficiency or lack of one or both of these proteins. Schwann cells are responsible for the myelination of axons in the PNS. A Schwann cell wraps itself, jelly roll-fashion, in a spiral around a short segment of an axon. During the wrapping, cytoplasm is squeezed out of the Schwann cell and the leaflets of plasma membranes of the concentric layers of the Schwann cell fuse, forming the layers of the myelin sheath. An axon's myelin sheath is segmented because it is formed by numerous Schwann cells arrayed in sequence along the axon. The junction where two Schwann cells meet has no myelin and is called (as you know) the node of Ranvier (the areas covered by Schwann cells being the internodal regions). The lack of Schwann cell cytoplasm in the concentric rings of the myelin sheath is what makes it lipid-rich. Schwann cell cytoplasm is however found in several locations. There is an inner collar of Schwann cell cytoplasm between the axon and the myelin, and an outer collar around the myelin. The outer collar is also called the sheath of Schwann or neurilemma, and contains the nucleus and most of the organelles of the Schwann cell. The node of Ranvier is also covered with Schwann cell cytoplasm, and this is the area where the plasma membranes of adjacent Schwann cells meet. (These adjacent plasma membranes are not tightly apposed at the node, so that extracellular fluid has free access to the neuronal plasma membrane.) Finally, small islands of Schwann cell cytoplasm persist within successive layers of the myelin sheath; these islands are called Schmidt-Lanterman clefts. The process of myelination is described in greater detail on pg. 264-268 of Ross et al. Not all nerve fibres is the PNS are covered in myelin, some axons are unmyelinated. In contrast to the situation in the CNS, unmyelinated fibers in the PNS are not completely bare, but are enveloped in Schwann cell cytoplasm. The Schwann cells are elongated in parallel to the long axis of the axons, which fit into grooves on the surface of the Schwann cell. One axon or a group of axons may be enclosed in a single groove. Schwann cells may have only one or up to twenty grooves. Single grooves are more common in the autonomic nervous system. An EM section of a node of Ranvier. Identify the axon, neurofilaments, plasma membrane, Schwann cell processes etc. Each axon is surrounded by myelin formed by a sequential series of Schwann cells. The myelin sheath shows gaps along its path called the nodes of Ranvier; these represent the spaces between adjacent Schwann cells along the length of the axon. Interdigitating processes of Schwann cells partially cover the node. The distance between two nodes is called an internode and consists of one Schwann cell. The length of the internode varies between 1 and 2 mm. Osmicated peripheral myelinated nerve There are no Schwann cells in the central nervous system; there, the myelin sheath is formed by the processes of the oligodendrocytes. Oligodendrocytes differ from Schwann cells in that different branches of one cell can envelop segments of several axons. • Schwann cells are the supporting cells of the peripheral nervous system (PNS), and are derived from neural crest cells. • Schwann cells form the myelin for axons and processes in the PNS. Each Schwann cell forms myelin for only a single internodal segment of a single axon. UNMYELINATED FIBERS In both the central and peripheral nervous systems, not all axons are sheathed in myelin. In the peripheral system, all unmyelinated axons are enveloped within simple clefts of the Schwann cells. Unlike their association with individual myelinated axons, each Schwann cell can sheathe many unmyelinated axons. Unmyelinated nerve fibers do not have nodes of Ranvier, because abutting Schwann cells - are united to form a continuous sheath. The central nervous system is rich in unmyelinated axons; unlike those in the peripheral system, these axons are not sheathed. In the brain and spinal cord, unmyelinated axonal processes run free among the other neuronal and glial processes. NONMYELINATED NERVE FIBRES Stained with hematoxylin and eosin • Unmyelinated axons in the PNS are enveloped by the cytoplasmic processes of a Schwann cell. • Schwann cells act as phagocytes and remove neuronal debris in the PNS after injury. • A node of Ranvier is the region between adjacent myelinated segments of axons in the CNS and the PNS. In all myelinated axons, nodes of Ranvier are sites that permit action potentials to jump from node to node (saltatory conduction). Saltatory conduction dramatically increases the conduction velocity of impulses in myelinated axons. DEGENERATION & REGENERATION OF NERVE TISSUE Although it has been shown that neurons can divide in the brain of adult birds, mammalian neurons usually do not divide, and their degeneration represents a permanent loss. Neuronal processes in the central nervous system are, within very narrow limits, replaceable by growth through the synthetic activity of their perikaryons. Peripheral nerve fibers can also regenerate if their perikaryons are not destroyed. Death of a nerve cell is limited to its perikaryon and processes. The neurons functionally connected to the dead neuron do not die, except for those with only one link. In this latter instance, the isolated neuron undergoes transneuronal degeneration. In contrast to nerve cells, neuroglia of the central nervous system—and Schwann cells and ganglionic satellite cells of the peripheral nervous system—are able to divide, by mitosis. Spaces in the central nervous system left by nerve cells lost by disease or injury are invaded by neuroglia. Since nerves are widely distributed throughout the body, they are often injured. When a nerve axon is transsected, degenerative changes take place, followed by a reparative phase. In a wounded nerve fiber, it is important to distinguish the changes occurring in the proximal segment from those in the distal segment. The proximal segment maintains its continuity with the trophic center (perikaryon) and frequently regenerates. The distal segment, separated from the nerve cell body, degenerates. Axonal injury causes several changes in the perikaryon: chromatolysis, i.e., dissolution of Nissl substances with a consequent decrease in cytoplasmic basophilia; an increase in the volume of the perikaryon; and migration of the nucleus to a peripheral position in the perikaryon. The proximal segment of the axon degenerates close to the wound for a short distance, but growth starts as soon as debris is removed by macrophages. Macrophages produce interleukin-1, which stimulates Schwann cells to secrete substances that promote nerve growth. In the nerve stub distal to the injury, both the axon (now separated from its trophic center) and the myelin sheath degenerate completely, and their remnants, excluding their connective tissue and perineurial sheaths, are removed by macrophages. While these regressive changes take place, Schwann cells proliferate within the remaining connective tissue sleeve, giving rise to solid cellular columns. These rows of Schwann cells serve as guides to the sprouting axons formed during the reparative phase. After the regressive changes, the proximal segment of the axon grows and branches, forming several filaments that progress in the direction of the columns of Schwann cells. Only fibers that penetrate these Schwann cell columns will continue to grow and reach an effector organ. When there is an extensive gap between the distal and proximal segments, or when the distal segment disappears altogether (as in the case of amputation of a limb), the newly grown nerve fibers may form a swelling, or neuroma, that can be the source of spontaneous pain. Regeneration is functionally efficient only when the fibers and the columns of Schwann cells are directed to the correct place. The possibility is good, however, since each regenerating fiber gives origin to several processes, and each column of Schwann cells receives processes from several regenerating fibers. In an injured mixed nerve, however, if regenerating sensory fibers grow into columns connected to motor end plates that were occupied by motor fibers, the function of the muscle will not be reestablished. • Demyelinating diseases are acquired conditions involving selective damage to myelin. Other diseases (e.g., infectious, metabolic, inherited) can also affect myelin and are generally called leukodystrophies. NERVE ENDINGS Specialized structures of nerve system, which lie at the beginning and the end of reflex arc. Classification of nerve endings I SENSORY (receptors) A. Disposition: 1. Interoceptors 2. Proprioceptors 3. Exteroceptors B. Feelings: 1. Pain 2. Pressure 3. Temperature C. Structure: 1. Simple (free) 2. Compound (nonfree): encapsulated, and noncapsulated II SYNAPSES (chemical and electrical) Structure Functions: excitatory, inhibiting Mediator: acetylcholine, adrenalin, bombesin III. EFFECTORY (effectors) Motor Secretory Sensory endings have dendrite inside. Reflex arch begins with receptor. Effectory endings are the last compound of reflex arch and consist of axon, which is connected with muscle (motor ending) or glandular cell (secretory ending). Synapses are specialized intercellular junctions of communicative type. Detail of a motor nerve ending upon a skeletal muscle cell (voluntary muscle). The axon terminal is highly branched to form an oval motor end plate. Myoneural junction The cell body which sends out this axon is a multipolar motor neuron, such as those in the anterior horn of the spinal cord. Electronmicrograph of axo-muscular synapse in skeletal muscle. X 33000. Diagram of motor end plate (myoneural junction) as seen with electron microscopy. This drawing shows a detail of one knob of an end plate as it rests in a trough on the surface of a muscle cell. The "subneural clefts" labelled here are also called "gutters" in the sarcolemmal membrane. The label "glycoprotein" indicates the position of the basal lamina of the muscle cell. EM detail of neuro-neural synapse in the brain or spinal cord. The axon terminal contains many seed-like synpatic vesicles containing transmitter substances. The intercellular cleft between the axon and the contacted dendrite can be seen. Just below the dendritic cell membrane is a dark, filamentous post-synaptic density. Other profiles in this field, most of them very irregular in outline, belong to both neuronal processes and glial processes. There is one large and one small mitochondrion just left of the synaptic vesicles. Muscle spindle – a specialized sensory receptor for muscle stretch and position sense, as related particularly to unconscious maintenance of skeletal muscle tone and proper balance of postural muscle activity. The spindle is the encapsulated group of muscle fibers lying in the center of the field of regular skeletal muscle fibers, all cut in cross-section. The sensory nerve endings themselves (not visible here) wrap around the muscle fibers within the spindle. Such endings relay sensory information along dendrites within peripheral nerves, back to pseudounipolar cell bodies in a spinal ganglion, and thence to the spinal cord. Pacinian corpuscle – another specialized sensory ending, this time for deep pressure. This particular view is from a whole mount of mesentery, so you are seeing the corpuscle three-dimensionally. They are also found in subcutaneous tissue, deep to skin. Notice the onion-like layers of specialized connective tissue surrounding a dark pink dendritic terminal. Again, the cell body for this dendrite lies in a spinal ganglion, and the axon of that same cell then proceeds into the spinal cord. Paccinian corpuscle Ruffini’s body (mechanoreceptor) Bulb of Crause (thermoreceptor) Meisner’s body (scheme) A Meissner's corpuscle. Seen bracketed, these are found unevenly distributed in the CT of the dermal papillae of the integument. This slide is from thick skin. These structures function in mediating light touch and tactile feeling. The arrow shows the dendritic nerve fiber, which spirals into a spindle-shaped structure in contact with the epidermis. The entire structure is ensheathed by a connective tissue capsule. STUDENT’S PRACTICAL ACTIVITIES Task No 1. Students must know and illustrate such a histologic specimens. Specimen 1. Non-nmyelinated nerve fibers Stained with hematoxylin and eosin. Under a low magnification, one must find the bands of nerve fibers stained in a pink color. Under a high magnification, they look like cords with oval shaped violet nuclei of neurolemmocytes along them. Axons lie inside the Shwann’s cells that is why they are invisible. Illustrate and indicate: 1. Unmyelinated nerve fibers. 2. Tunica. 3. Shwann’s cells nuclei. 4. Axons. Specimen 2. Myelinated nerve fibers Stained with osmic acid. Under a low magnification, one must find myelinated fibers disposed in different turns and choose isolated one. Under a high magnification, one must watch a pale stained axon in the middle of the nerve fiber. A black colored myelin surrounds it. Find thin light lines in the myelin sheath that have oblique turns – Schmidt-Lanterman clefts and the other portion without myelin – Ranvier node. Neurolemma is a thin light tunica around the nerve fiber. Illustrate and indicate: 1. Axon. 2. Myelin. 3. Ranvier nodes. 4. Myelin incisures. 5. Neurolemma. Specimen 3. Lamellar (Fatter-Paccinian) corpuscle. Stained with haematoxylin and eosin. Under a low magnification, one must find in derma a round or an oval-shaped Paccinian body with terminal branches of axon in the middle portion that is surrounded by changed lemmocytes (inner bulb). There are concentrically disposed connective tissue lamellae with fibroblasts around it (external bulb). Illustrate and indicate: Inner bulb. 2. Axons branches. 3. Outer lamellae of the corpuscle. 4. Connective tissue lamellae. 5. Fibroblasts nuclei. Task No 2. Students must know and illustrate such a scheme. Synapse scheme. Illustrate and indicate: Presynaptic pole: a) synaptic vesicles; b) mitochondria; c) presynaptic membrane. Synaptic fissure. 3. Postsynaptic pole: a) postsynaptic membrane; b) mitochondria Task No 3. Students should be able to indicate elements in the electron micrographs: 1. Fragment of the neuron 2. Neurosecretory cell 3. Oligodendrocyte 4. Astrocyte (cerebral cortex) 5. Ependimal cells 6. Myelinated nerve fiber 7. Non-myelinated nerve fiber 8. Synapse. Interneuronal junction 9. Axo-vasal synapse in neurohypophisis 10. Motor nerve ending (myoneural junction) References: A-Basic: 1. Practical classes materials http://intranet.tdmu.edu.ua/data/kafedra/internal/histolog/classes_stud/English/ medical/II%20term/10%20Nerve%20tissue.%20Nerve%20cells.%20Glial%20c ells.%20Nerve%20fibers.%20Nerve%20endings.htm 2. Lecture presentations http://intranet.tdmu.edu.ua/ukr/kafedra/index.php?kafid=hist&lengid=eng&faku ltid=m&kurs=1&discid=Histology, cytology and embryology 3. Stevens A. Human Histology / A. Stevens, J. Lowe. – [second edition]. – Mosby, 2000. – P.77-82 4. Wheter’s Functional Histology : A Text and Colour Atlas / [Young B., Lowe J., Stevens A., Heath J.]. – Elsevier Limited, 2006. – P. 122-150 5. Inderbir Singh Textbook of Human Histology with colour atlas / Inderbir Singh. – [fourth edition]. – Jaypee Brothers Medical Publishers (P) LTD, 2002. – P. 135-167 6. Ross M. Histology : A Text and Atlas / M. Ross W.Pawlina. – [sixth edition]. – Lippincott Williams and Wilkins, 2011. – P. 352-400 7. Kaplan Medical. USMLE Step 1 Lecture Notes 2018: Pathology, Pharmacology, Physiology, Biochemistry and Medical Genetics, Immunology and Microbiology, Anatomy, and Behavioral Science/Social Sciences / Kaplan Medical., 2018. – 2567 р. B - Additional: 1. Eroschenko V.P. Atlas of Histology with functional correlations / Eroschenko V.P. [tenth edition]. – Lippincott Williams and Wilkins, 2008. – P. 135-156 2. Junqueira L. Basic Histology / L. Junqueira, J. Carneiro, R. Kelley. – [seventh edition]. – Norwalk, Connecticut : Appleton and Lange, 1992. – P. 152-168 3. Volkov K. S. Ultrastructure of cells and tissues / K. S. Volkov, N. V. Pasechko. – Ternopil : Ukrmedknyha, 1997. – P. 82-90 Information resources: 1. https://moodle.tdmu.edu.ua/ 2. http://www.histologyguide.com/ 3. http://www.cytology.net/ 4. https://web.duke.edu/histology/ 5. https://www.meyershistology.com/ 6. https://histology.sites.uofmhosting.net/full-slide-list 7. http://en.wikipedia.org/wiki/Histology 8. http://217.196.164.19/data/books/Volkov(atlas).pdf 9. http://www.udel.edu/biology/Wags/histopage/histopage.htm 10. http://217.196.164.19/data/books/Volkov(atlas).pdf 11. http://en.wikipedia.org/wiki/Circulatory 12. http://www.meddean.luc.edu/LUMEN/MedEd/Histo/frames/histo_frames.html 13. http://www.udel.edu/biology/Wags/histopage/histopage.htm