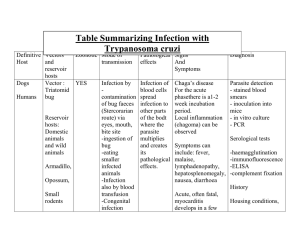
I. BACKGROUND AND TERMINOLOGIES A. PARASITES – organisms that live on and obtain their nutrients from another organism B. PARASAITOLOGY – study of parasites C. INFECTION - parasites invading in the body D. INFESTATION – invading on the body E. DISEASE - process with characteristic symptoms, emerged, determining an effective means of healing infected people F. VECTORS – transport carriers G. MODE OF TRANSMISSION - the means whereby a parasite enters an unsuspecting host IV. II. PARASITE-HOST RELATIONSHIPS - MAIN FOCUS OF THIS RESEARCH: 1. Recognition of these relationships 2. Search for patterns 3. Development of methodologies to study these patterns SEE TABLE 1-1 III. PARASITIC LIFE CYCLES - 3 MAIN COMPONENTS: 1. Mode of transmission 2. Infective stage 3. Diagnostic stage - 2 COMMON PHASES: 1. Route a parasite follows when in or on the human body 2. Route a parasite follows independently of the human body V. DISEASE PROCESSES AND SYMPTOMS - MAJOR AREAS AFFECTED: 1. GI and urogenital tracts 2. Blood and tissue 3. Liver, lung, and other major organs 4. Miscellaneous locations (e.g. eye, skin, etc.) - SYMPTOMS ASSOCIATED WITH PARASITIC DISEASE PROCESSES 1. Diarrhea 2. Fever 3. Chills 4. Abdominal pain and cramping 5. Elephantiasis (accumulation of lymph) 6. Anemia 7. Vitamin deficiency 8. Bowel obstruction 9. Edema 10. Enlargement of major organs 11. Skin lesions 12. Blindness TREATMENT - PARASITE TREATMENT OPTIONS a. Antiparasitic medications b. Change in diet c. Vitamin supplements d. Fluid replacement e. Blood transfusion f. Bed rest VI. VII. VIII. PREVENTION AND CONTROL - Designed to break the transmission cycle are crucial for successful parasite eradication - Some strategies: a. Development and implementation of parasite awareness education programs b. Use of insecticides and other chemicals c. Use of protective clothing d. Use of protective netting e. Proper water treatment f. Good personal hygiene g. Proper sanitation practices h. Proper handling, cooking, and protection of food i. Avoidance of unprotected sexual relations SPECIMEN PROCESSING AND LABORATORY DIAGNOSIS 1. O&A – aka ova (eggs) and parasites 2. Giesma stain – blood (sample for parasite study) 3. Cellophane tape preparation – pinworm eggs 4. Enterotest (string test) – recovery of several parasites PARASITE NOMENCLATURE AND CLASSIFICATION 1. Written in italics 2. Genus + species 3. Variations of genus names are used to denote the disease associated with the parasite 4. When first appeared in documents, the whole name is written out 5. -iasis → denotes diseases/conditions - 3 MAJOR GROUPS OF CLINICALLY SIGNIFICANT PARASITES: 1. Single-celled parasites (protozoa) 2. Multicellular worms (metazoan helminths 3. Arthropods (animalia) Specimen Collection and Processing • • • • • Collection and Transportation Fixatives for Preservation Processing Stool Screening Methods Other Specimens and laboratory techniques COLLECTION AND TRANSPORT • Protozoan forms (trophozoites and cysts) and helminth stages (egg, larvae, proglottids, and adult worms) are found in stool samples. • Typical stool collection protocol: o 3 specimens in 10 days • Amebiasis o 6 specimens in 14 days SAMPLE LABELLING: • Patient’s name • Identification number • Physician’s name • Date and time of collection • Age • Gender ➢ Trophozoite motility – fresh sample is required. ➢ Protozoan cysts helminth egg and larvae are not sensitive. ➢ Liquid samples: Processed within 30 mins. (Presence of trophozoites) ➢ Semi-formed stool samples: Evaluated within 1 hour (Presence of trophozoites and cysts) ➢ Formed samples: Can be held within 24 hours. ➢ Guidelines are not met; the samples must be preserved. FIXATIVE AS PRESERVATION: • Ideal Sample: Freshly collected stool sample • Substances that preserve the morphology of protozoans and prevent development of helminth eggs and larvae. • Ratio is 3:1 (3 parts fixative; 1 part of stool) 1. Fomalin 2. Polyvinyl Alcohol (PVA) 3. Sodium Acetate Formalin (SAF) 4. Modified Polyvinyl Alcohol 5. Alternative Single Vial Systems 1. FORMALIN • All-purpose fixative for helminths and protozoans. • 2 concentrations: o 5% - protozoan cysts o 10% - helminth eggs and larvae. • Used for routine direct examinations and concentration techniques but NOT for permanent smears. ADVANTAGES: • Easy to prepare • Preserves specimen up to several years. • Long shelf-life DISADVANTAGES: • Does not preserve parasite morphology adequately for permanent smears. • Trophozoites may not be recovered. • Morphology of cysts and eggs may fade with time. • Potential health hazard. 2. POLYVINYL ALCOHOL (PVA) • Combined with Schaudinn solution (Zinc Sulfate, Copper Sulfate or Mercuric Chloride). ADVANTAGES: • Choice of permanent stain (Iron Hematoxylin) 4. MODIFIED POLYVINYL ALCOHOL (PVA) • Alternative fixative to Mercurybased PVA by using Copper Sulfate and Zinc Sulfate. • Long shelf-life when stored at room temperature. • Can be used for concentration methods and permanent smears. • Concentration techniques can be performed (Two-vial system). • Do not provide the same quality of preservation of protozoan morphology. • Trophozoites and cysts of protozoans, most helminth eggs can be detected. • Can be used for preparation in a permanent stained smear. DISAVANTAGES: • Potential health problems (Mercury in Schaudinn solution). 3. SODIUM ACETATE FORMALIN (SAF) • Alternative fixative to PVA and Schaudinn fixative. • Used in concentration techniques and permanent stained smears. ADVANTAGES: • Easy to prepare. • Long-shelf life • Use for preparing smears for staining with modified acid-fast stain for Coccidian cysts. DISADVANTAGES: • Addition of Albumin is necessary. • Protozoan morphology is not clear in permanent smears. 5. ALTERNATIVE SINGLE-VIAL SYSTEM • Single vial systems are free of Formalin and Mercury. • Used for concentration techniques and permanent smears. • Used in fecal immunoassays. • Disadvantage: 1. Do not provide the same quality as Mercury-based fixative. 2. Organism identification will be more difficult. PROCESSING MACROSCOPIC - Consistency (degree of moisture) - Color (Normal: brown) - Gross abnormalities (adult worms, proglottids, pus and mucus) MICROSCOPIC - Direct methods - Concentration techniques - Permanently stained smears MICROSCOPIC EXAMINATION: 1. Direct Wet Preparation 2. Concentration methods 3. Permanent Stained slides • DIRECT WET PREPARATION • “Direct Wet Mount” • Slide made by mixing a small portion of unfixed stool with saline or Iodine. • To detect the presence of motile protozoan trophozoites (fresh samples). • Direct Saline Wet Preparation • Direct Iodine Wet Preparation • Debris sinks at the bottom of the tube. • Parasites are lighter thus rises on the top of the tube. • Zinc sulfate (1.18-1.20) is used as the concentrating solution. ADVANATAGES: • More fecal debris is removed. • Cleaner preparation, easier for microscopic examination. • CONCENTRATION TECHNIQUES: FORMALIN-ETHYL ACETATE SEDIMENTATION - Principle: Specific gravity - Ethyl Acetate is added to a salinewashed formalin-fixed sample. - Parasites heavier than the solution settles in the sediment of the tube. - Fecal debris are lighter and rises to the upper layer of the tube. ADVANTAGES: • Easy to prepare • Good recovery of parasites DISADVANTAGES: • More fecal debris • Challenging to microscopist. ZINC SULFATE FLOTATION • Principle: Specific gravity DISADVATAGE: • Some helminths are dense and will not float. • PERMANENT STAINS • Final procedure in O&P examination. • Microscope slides that contains a fixed sample that has been allowed to dry and stained. • Critical portion of the O&P examination. • Sample of choice is PVA-prepared sample. SAF samples can be used but the stain must be Iron Hematoxylin. • Slides can be prepared from fresh samples but must not be allowed to dry and place immediately into a fixative. • Two common stains: o WHEATLY TRICHROME o IRON HEMATOXYLIN WHEATLY TRICHROME • Most widely used permanent stain. • Long shelf life and easy to perform. • Distinct color differenced between the cytoplasmic and nuclear structures. IRON HEMATOXYLIN • Excellent morphology of intestinal protozoa. • Nuclear detail are stained clearer and sharper. SPECIALIZED STAINS • Modified acid-fast stain (Cryptosporidum, Isospora, Cyclospora spp.) • Do not detect oocysts and spores of (Coccidian/Sporidia) • Modified Iron Hematoxylin (incorporates with Carbolfuchsin to detect acid-fast parasites; combined with SAF-preserved fecal samples.) STOOL SCREENING METHODS • “Rapid methods” (obtained as kits that contain monoclonal antibody). • Commercial antibody is used to detect antigens in patient’s samples. • Enzyme Immunoassay, DFA & Membrane Flow Cartridge Technique. • E. histolytica, G. intestinalis & Cryptosporidum spp. • Highly sensitive and specific but detects one pathogen at a time. PART II • Duodenal material • Sigmoidoscopy material • Cellophane type preparation DUONENAL MATERIAL • Collected by nasogastric intubation or by Enteric capsule test (Enterotest). • G. intestinalis troph, Cryptosporidum spp., Isospora belli, Strongloides stercoralis and Clonorchis sinensis/ Fasciola hepatica. • Examined by wet prep but if >2mL it can be centrifuged, and sediments examined. • Mixed with PVA fixative and stained using Trichome, Iron Hematoxylin and/or Modified Acidfast. • Antigen test: G. intestinalis & Cryptosporidum spp., CELLOPHANE TAPE • Detection of Enterobius vermicularis & Taenia spp. • Specimen is collected in the morning. • Proper hygiene, PPEs during specimen collection to avoid spreading of the infection. SIGMOIDOSCOPY MATERIAL • Colon • Detecting Entamoeba histolytica and Coccidian spp. • Materials from ulcers obtained by scrapings or aspirations must be examined by wet preparation and permanent stains. OTHER SPECIMENS AND LABORATORY TECHNIQUES BLOOD • Leishmania donovani & Trypanosoma cruzi, Plasmodium spp., Babesia spp. • Trypanosoma cruzi & Mircofilariae can be detected by observing the motility in a wet preparation of a fresh blood sample. • Can be processed by: - Knott technique • Blood smears are prepared from fresh whole blood without the anticoagulant (fingertip/ earlobe)/ venipuncture collection with anticoagulant. - Buffy coat slides - Cultures COLLECTION AND HANDLING • Blood collection is by aseptic technique. • Site for puncture: Earlobe / fingertip • Capillary blood must be freeflowing, not contaminated with alcohol. • Anticoagulants can cause distortion to staining thus affecting the morphology. • Blood specimens are collected in EDTA tubes. • MALARIA – smears are prepared within 1hour. • Timing of obtaining blood samples varies. THICK SMEARS: • • • • PROCESSING: Satisfactory for SCREENING. Dehemoglobinized (more conc.) Few in number / thin smears (-) Inreased in detection of malarial parasites • RBCs lysed (poor morphology) • Blood sample processing: THIN SMEARS: - Preparing thick and thin smears - Staining using permanent smears - Microscopic examination • BEST VIEW of Malarial parasites in RBCs • Recommended for species identification 5. Stain the sediment with Giemsa. PERMANENT STAINS: WRIGHT’S STAIN: • Contains the fixative and stain in one solution. • Yields satisfactory results GIEMSA STAIN: • The fixative and stain are separate. • Preferred stain because it allows detection of parasite detail necessary for species identification. KNOTT TECHNIQUE: • Designed to concentrate blood samples suspected with Microfilariae. • Procedure: 1. Combine 1mL of blood with 10mL of 2% Formalin in a centrifuge tube. 2. Mixture will thoroughly mixed. 3. Spun for 1 min. at 500 x g. 4. Thick slides are made and dried. BUFFY COAT SLIDES: • Layer of WBCs between plasma and RBCs (centrifuged). • Extracted from blood specimens and stained with Giemsa (Leishmania & Trypanosoma). • Procedure: 1. Collected oxalated/ citrated blood and place it in a Wintrobe tube. 2. Spin for 30 mins at 100xg (tubes are capped tightly). 3. Centrifugation produces 3 layers: Packed RBCs, buffy coat and plasma. 4. Extract the buffy coat using a capillary pipette. CULTURES: • Blood culture as well as bone marrow and tissue. • Favorable results for the recovery of Leishmania spp. & Trypanosoma cruzi. • It uses Novy-MacNeal-Nicolle (NNN) medium. • Negative cultures are held for 1 month. • Procedure: • NNN slant is inoculated by adding a drop of blood/ tissue. • Penicillin is added. • Periodic examination under 400x CEREBROSPINAL FLUID • Diagnosis of Amebic conditions (African Sleeping Sickness) • Wet preparation (Naegleria fowleri & Acanthamoeba spp., Trypanosoma spp.) • Giemsa, Trichrome and Modified Trichrome stain is used. • If Naegleria & Acanthamoeba spp. are suspected: - Cultured on Non-Nutrient Agar seeded with E. coli. - CSF sediment is inoculated, sealed and incubated at 35 - Examine plate, the amoeba feeding on the the bacteria. • Other parasites: Toxoplasma gondii, Microsporidia, Taenia solium cysticerus larvae and Echinococcus spp. OTHER STERILE FLUIDS: • Cystic fluid • Aspirates • Peritoneal fluid • Pleural fluid • Bronchial washings TISSUE AND BIOPSY SEPCIMEN • Recovery of intracellular parasites: Leishmania spp. & Toxoplasma gondii. • Free-living amoeba, Trypanosoma spp., Trichinella spiralis & Microsporidia • Hepatic abscess: E. histolytica SPUTUM: • Collected and tested from patients suspected with Lung fluke: Paragonimus westermanii • Hyperinfection by Strongyloides stercoralis • Other parasitic infections: Microsporidia, E. histolytica, E. gingivalis, A. lumbricoides and hookworms. • Early-morning specimen is best and should be collected in a mouth-wide container with screwcap lid. • Saliva must not be mixed with the sputum. • Examined directly by wet preps/ conc. N-acetylcysteine. URINE AND GENITAL SECRETIONS: • Urine – specimen of choice for Schistosoma haematobium & Trichomonas vaginalis • Vaginal, urethral and prostatic excretions are examined for T. vaginalis • Specimens are collected on a swab/ collection cup. • Saline wet preparation is the method of choice. • Latex Agglutination and EIA – T. vaginalis • Culture methods: Diagnosis of STDs EYE SPECIMENS: • Corneal scrapings: Acanthamoeba keratitis • Other parasites: T. gondii, Loaloa, Microsporidia • Collection: 1. Scraping are collected in airtight container. 2. Kept moist with Saline solution. 3. Other specimens: Contact lens/ contact lens solution 4. Can be cultured in an agar plate with gram-negative bacteria. 5. Scrapings can be transferred into glass slides, stained using Calcofluor white and examined under Fluorescent microscopy. (Acanthamoeba cysts stain apple green) 6. Scrapings can be processed using histologic methods. MOUTH SCRAPING AND NASAL DISCHARGE: • Mouth scaping is the specimen of choice for E. gingivalis and T. tenax. • Nasal discharged is the specimen of choice for Naegleria fowleri. • Both samples are placed in a clean, airtight container (swab/cup) 1. Obtain skin fluid without bleeding. 2. Material obtained is placed on 0.2mL of Saline. 3. Incubate for 30 mins. 4. Read microscopically, observe the jerky movement of the microfilariae. • Can be both prepared using wet preps and permanent stains. CULTURE METHODS: • Not a common means of detecting parasites. SKIN SNIPS: • Useful in detection of Onchocerca volvulus. • Procedure: • Special laboratories and research facilities may offer these services. • Parasites that can be cultured: - Entamoeba histolytica - Trichomonas vaginalis - Leishmania spp. - Trypanosoma cruzi - Toxoplasma gondii ANIMAL INOCULATION AND XENODIAGNOSIS: • Appropriate specimens from patients suspected with Leishmaniasis, Trypanosomiasis and Toxoplasmosis may be tested using animal inoculation. • Mice, guinea pigs and hamsters are used. IMMUNOLOGIC TESTING: • Methods of antigen and antibody detection. • Antigen detection methods are more reliable and positive result is indicative of current infection. • Positive antibody tests are indicative of past infection. • Not usually offered in routine laboratories and specimens might be sent out in reference laboratories. • Nucleic acid tests are developed. • Specimens: blood, lymph nodes, CSF and bone marrow. • Only commercial molecular test available is for T. vaginalis. • Collected using aseptic technique. RESULT AND QUALITY CONTROL: • Post-analytic phase of the laboratory testing. • Xenodiagnosis – used for Chagas’ disease. - Uninfected reduviid bug can take a blood meal from a patient, the bug’s feces is examined to observe the presence of T. cruzi. - Used in South America and Mexico. • Positive specimen: - State the scientific name (genus and species) ex. Entamoeba histolytica - Include the stage: cysts, trophozoites, larvae, eggs and adults - Report cells: Presence of WBCs (semiquantitatively: rare, few, moderate, many) • Results of O&P: - Include a comment regarding the limit of the test procedures. • Fecal Immunoassay: - Indicate the specific parasite that test for the assay. - Schistosoma spp. - Plasmodium spp. - Babesia spp. • Charcot-Leyden crystals are also reported and quantitated when seen. QUALITY ASSURANCE: • Consistent with Microbiology laboratory. - Procedure manuals are up to date and readily available. - Reagents and solutions must be labeled, controls in concentration. - Stains, centrifuges, micrometers must be calibrated. - Refrigerator and incubator temperatures must be recorded. • Quantitation of parasites is not indicated. • Quantitation is important when these parasites are observed: - Blastocystis hominis - Trichuris trichiura - Clonorchis sinensis - References for laboratory training. - Must also include texts, atlases, digital images, reference samples - Participate in External quality and internal proficiency programs WATER SAMPLES: • Detection of Cryptosporidium sp. and Giardia sp. in bulk water samples. • FLA (Free-Living Amoeba) • Cryptosporidium sp. or Giardia sp. is seeded on NNA with E. coli. MOLECULAR TESTING: 1. DNA Extraction 2. Polymerase Chain Reaction (PCR) 3. Agarose Gel Electrophoresis SOIL SAMPLES: • Rich reservoir for protozoans and helminths. • Popular choice for detection of infective stages of hookworms (filariform larvae). • Collected through and Auger (soil tube). DNA EXTRACTION: • Extracting the DNA in the nucleus of the target organism. STEP 2: PRECIPITATION • Commercial kits: QIAGEN, Mcherey Nagel • Achieved by: • FLA: Simple extraction (application of heat). • Toxoplasma sp. – additional method such as freeze-thaw cycles. • Helminths – Sonification/ freezethaw cycle. • Separate the DNA from cellular debris. ✓ Addition of Na+ ions (makes the DNA more stable and less water soluble). ✓ Alcohol (Ethanol/ Isopropanol) – causes the DNA precipitate out of the solution since it is alcohol insoluble. STEP 3: PURIFICATION • Rinsing the precipitated DNA with alcohol to remove unwanted cellular debris. • DNA is dissolved in aqueous solution (ultrapure water). STEP 1: LYSIS • Destruction of the cellular and nuclear wall to liberate DNA. • Achieved by: ✓ Lysis through detergents or enzymes (Proteinase K). ✓ Mechanical means by homogenizer, mortar and pestle. • Tissue sections – cutting into smaller pieces 2. POLYMERASE CHAIN REACTION (PCR): • Most frequently used technique. • Achieved by using Thermal cycler. • Purpose: Multiply several copies of the target gene by priming complementary sequences. STEP 1: INTIAL DENTURATION • Double-helix strands of the target DNA are uncoiled and separated. • Temperature: 90 to 95. STEP 2: ANNEALING • Also known as Hybridization • Primers bind to their complementary bases on each single strand of DNA. • Primer sets will bind in the beginning and end of the chosen target gene. • Temperature: 55 - DNA is negatively charged and will travel to the positive region when electric current is applied. - Migration speed depends on its molecular weight. • Comparison: - DNA ladder is loaded into the first well. • Done at 75 - Ethidium Bromide or nontoxic gel dye is added (better visualization). • Polymerase read the template strands and matches the nucleotides. - Buffers used are AGE Preps (Tris-Acetic acid-EDTA buffers). STEP 3: POLYMERIZATION • Results to formation of two helical strands. ✓ One from the original strand. ✓ One from the complementary strand. • Repeated 30 to 40 times. 3. AGAROSE GEL ELCTROPHOROSIS • Principle: - Charged particles is made to travel in a gel medium submerged in a buffer solution. 1.1 MORPHOLOGY AND LIFE CYCLE NOTES - Equipped with the ability to extend their cytoplasm in the form of pseudopods (false feet) - 2 MORPHOLOGIC FORMS IN THE AMEBIC LIFE CYCLE: 1. TROPHOZOITES - form that feeds, multiplies, and possesses pseudopods - Delicate and fragile - Motile (can produce and use pseudopods) - Easily destroyed by the gastric juices of the stomach - Not usually transmitted to humans - EXCYSTATION – cyst to trophozoite (occurring in the ileocecal area of the intestine) - ENCYSTATION – trophozoites to cysts; triggered by a) ameba overpopulation, b) pH change, c) food supply, d) available oxygen 2. CYSTS – nonfeeding stage characterized by a thick protective cell wall designed to protect the parasite from the harsh outside environment 1.2 LABORATORY DIAGNOSIS - Trophozoites → soft, liquid, or loose consistency stools - Cysts → formed stools - SALINE WET PREPARATIONS – show motility of the amebic trophozoites - IODINE WET PREPARATIONS – better seen internal cytoplasmic and nuclear structures - Permanent smear procedures of sample suspected of having amoebas must be performed to confirm parasite identification 1.3 PATHOGENESIS AND CLINICAL SYMPTOMS - A number of patients infected with intestinal amoebas are asymptomatic. - In the United States, amebiasis is often found in immigrants from and people who have traveled to underdeveloped countries. 1.4 CLASSIFICATION OF THE AMEBAS - Separated into two categories: 1. Intestinal 2. Extraintestinal – parasites that migrate and/or take up residence outside the intestines A. Entamoeba histolytica (intestinal) B. Entamoeba hartmanni (intestinal) C. Entamoeba coli (intestinal) D. Entamoeba polecki (intestinal) E. Entamoeba nana (intestinal) F. Iodamoeba butschlii (intestinal) G. Entamoeba gingivalis (extraintestinal) H. Naegleria fowleri (extraintestinal) I. Acanthamoeba species (extraintestinal) AMOEBA Entamoeba histolytica MORPHOLOGY LABORATORY DIAGNOSIS Antigen tests Enzyme-linked immunosorbent assay (ELISA) Indirect hemagglutination (IHA) Gel diffusion precipitin (GDP) Indirect immunofluorescence (IIF) Serologic tests (helpful in cases of extraintestinal infections) LIFE CYCLE NOTES EPIDEMIOLOGY CLINICAL SYMPTOMS Excystation occurs in the small intestine. Nuclear division → cyst producing 8 motile trophozoites → settles in lumen of colon → binary fission → feeding on living host cells Affects 10% of world’s population ASYMPTOMATIC CARRIER STATE: 1. Parasite is a low-virulence strain 2. Inoculation into the host is low 3. Patient’s immune system is intact SYMPTOMATIC INTESTINAL AMEBIASIS 1. Amebic colitis 2. Amebic dysentery SYMPTOMATIC EXTRAINTESTINAL AMEBIASIS: 1. Abscess 2. Amebic pneumonitis 3. Cough, weakness, weight loss, sweating, sweating, nausea and vomiting 4. Venereal amebiasis Leading cause of parasitic deaths after only malaria Thrives in subtropical and tropical areas Ingestion of the infective stage, the cyst, occurs through hand-to-mouth contamination and food or water contamination. Unprotected sex, vectors (e.g. flies and cockroaches) and improperly treated water supplies are possible source of infection. TREATMENT: paromomycin, diloxanide furoate (Furamide), or metronidazole (Flagyl) → asymptomatic iodoquinol, paromomycin, or diloxanide furoate → symptomatic intestinal amebiasis Metronidazole or tinidazole → extraintestinal amebiasis PREVENTION AND CONTROL Boiling water with iodine crystals (infective (quadrinucleated) cyst is resistant to routine chlorination) filtration and chemical treatment of water properly washing food products avoiding the use of human feces as fertilizer goof personal and sanitation practices protection of food from flies and cockroaches avoidance of unprotected sexual practices AMOEBA Entamoeba hartmanni MORPHOLOGY LABORATORY DIAGNOSIS Examining stool Use of ocular micrometer is needed for size identification EPIDEMIOLOGY Geographic distribution is cosmopolitan ingestion of infected cysts present in contaminated food or water accounts for the transmission of E. hartmanni CLINICAL SYMPTOMS ASYMPTOMATIC TREATMENT - Nonpathogen - Treatment not indicated PREVENTION AND CONTROL Good sanitation and personal hygiene practices Protection of food from flies and cockroaches AMOEBA Entamoeba coli MORPHOLOGY LABORATORY EPIDEMIOLOGY DIAGNOSIS Stool Found worldwide examination Occurs in cold climates Geographic areas that have poor hygiene and sanitation practices Transmitted through the ingestion of the infected cyst through contamination food or drink CLINICAL SYMPTOMS ASYMPTOMATIC TREATMENT - Nonpathogenic - Not indicated PREVENTION AND CONTROL adequate disposal of human feces proper personal hygiene practices Protection of food and drink from flies and cockroaches AMOEBA Entamoeba polecki MORPHOLOGY LABORATORY DIAGNOSIS Stool samples EPIDEMIOLOGY Considered a parasite of pigs and monkeys Human infections are relatively rare Ingestion of this cyst is most likely responsible for the onset of infection CLINICAL SYMPTOMS ASYMPTOMATIC Discomfort associated – diarrhea TREATMENT - Metronidazole (flagyl) and diloxanide furoate (Furamide) PREVENTION AND CONTROL Improving personal hygiene and sanitation process AMOEBA Endolimax nana MORPHOLOGY LABORATORY DIAGNOSIS Stool samples EPIDEMIOLOGY Warm, moist regions CLINICAL SYMPTOMS ASYMPTOMATIC Poor hygiene and TREATMENT: substandard sanitary - nonpathogen conditions Contaminated food and water PREVENTION AND CONTROL Protection of food and drink from flies and cockroaches Good sanitation and personal hygiene AMOEBA Iodamoeba butschlii MORPHOLOGY LABORATORY DIAGNOSIS EPIDEMIOLOGY Stool samples Higher prevalence in tropical regions than in temperate regions Method of diagnosis: 1. fecalysis 2. concentration techniques 3. molecular techniques iodine wet preps – benefits in identifying cysts glycogen mass remains unstained following trichrome staining Lower frequency in transmission of E. coli and E. nana Transmission of I. bütschlii occurs when the infective cysts are ingested in contaminated food or drink. Hand-to-mouth transmission may also occur CLINICAL SYMPTOMS PREVENTION AND CONTROL Nonpathogenic Personal hygiene and Does not sanitation produce clinical symptoms AMOEBA MORPHOLOGY Entamoeba gingivalis LABORATORY DIAGNOSIS LIFE CYCLE NOTES EPIDEMIOLOG Y CLINICAL SYMPTOMS Mouth scrapings Lives around the gum line of the teeth in the tartar and gingival pockets Contracted via mouth-to-mouth Infections in the mouth and in the genital tract produce no symptoms Material from the tonsillar crypts and pulmonary abscess Sputum No known cyst stage Vaginal and cervical material Its trophozoites inhabit tonsillar crypts and bronchial mucus Trophozoites feed on disintegrated cells and multiply by binary fission Higher chance of infection to women who have IUDs Droplet contamination (transmitted through contaminated drinking utensils) Nonpathogenic E. gingivalis trophozoites are frequently recovered in patients suffering from pyorrhea alveolaris. do not produce symptoms of their own TREATMENT: - not indicated (nonpathogenic) PREVENTI ON AND CONTROL Proper care of the teeth and gums Prompt removal of IUDs AMOEBA MORPHOLOGY Naegleria fowleri LABORATORY DIAGNOSIS LIFE CYCLE NOTES EPIDEMIOLOGY CLINICAL SYMPTOMS Cerebrospinal fluid (saline and iodine wet preps) Ameboid trophozoites of N. fowleri are the only form known to exist in humans. Warm bodies of water (e.g. lakes, streams, ponds and swimming pools) Samples of tissue and nasal discharge CYST: - 9 to 12 µm - generally round and have thick cell walls - one nucleus with a large centrally located karyosome (no peripheral chromatin) - granular and with vacuole cytoplasm May be cultured (has trailing effect when placed on agar plates that have been previously inoculated with gram-negative bacili Replicate by binary fission Trophozoites converting to cysts and flagellates and then back to amebic trophozoites, occurs in the external environment Contaminated ducts Nasal mucosa In the passages → Asymptomatic PAM → invade the brain → rapid tissue destruction. Symptoms: fever, headache, sore throat, nausea, and vomiting. Stiff neck and seizures, smell and taste alterations, blocked nose and Kernig’s sign. 3-6 days after onset → death. TREATMENT: Amphotericin B, rifampin, or miconazole. PREVENTION AND CONTROL Total eradication is highly unlikely Posting offlimits signs Educating the medical community Chlorination of pools and hot tubs AMOEBA Acanthamoeba species MORPHOLOGY LABORATORY DIAGNOSIS LIFE CYCLE NOTES EPIDEMIOLOGY CLINICAL SYMPTOMS CSF examination, brain tissue Acquired by: 1. aspiration or nasal inhalation 2. direct invasion of the parasite in the eye (affects those who wear contact lenses and those who have experience d trauma to the cornea) Primarily occur in patients who are immunocompromise d or debilitated Granulomatous amebic encephalitis: - headaches - seizures - stick neck - nausea - vomiting - granulomatous lesions Corneal scrapings → choice of recovery (eye) [may be cultured on non-nutrient agar plates seeded with gram-negative bacteria] Indirect immunofluorescen t → choice for speciating Method of diagnosis: 1. microscopy 2. culture methods (NNA lawned with E. coli) 3. molecular methods Migrate through hematogenous spread and invade the CNS Acanthamoeba keratitis → serious eye infections that is cause by contact lenses who use homemade, nonsterile saline solutions Immunocompromise d animals appear to contract fatal CNS infections Acanthamoeba keratitis: - amebic keratitis - sever ocular pain - vision problems - perforation of the cornea TREATMENT: - sulfamethazine , itraconazole, ketoconazole, miconazole, propamidine isethianate, and rifampin. PREVENTIO N AND CONTROL Follow manufacturerestablished protocols Nonsterile saline solutions BACKGROUND - Phylum protozoa - Subphylum Mastigophora MORPHOLOGY - FLAGELLA – responsible for its movement (whiplike structures) - All flagellate life cycles consist of the trophozoite form - Those with no known cyst stage – more resistant to destructive forces, surviving passage into the stomach following ingestion - Reside mainly in the small intestine, cecum, colon and duodenum - FLAGELLATE CYSTS – equipped with thick, protective cell walls (may survive in the outside environment) LABORATORY DIAGNOSIS - Stool examination - TROPHOZOITES – loos, liquid, or soft stool - CYSTS – formed stools - Presence of either or both flagellate morphologic forms is diagnostic - Parts that help with flagellate identification: 1. AXOSTYLE – a rodlike support structure found in some flagellates 2. Undulating membrane - Flagellate trophozoites – flagella is not always visible - Stains used: 1. Saline 2. Iodine wet preparations 3. Permanent stains PATHOGENESIS AND CLINICAL SYMPTOMS - Often recovered from patients suffering from diarrhea without an apparent cause - Pathogenic flagellates have transmission routes similar to those of the non-pathogenic variety - G. intestinalis – the only intestinal flagellate, pathogenic Flagellate Giardia intestinalis Morphology Lab diagnosis Stool examination Shed in showers – many organisms may be passed and recovered on one day’s sample and the ff day’s sample ay reveal no parasites at all Multiple samples is recommended Tests used: 1. Enterotest (string test) 2. duodenal contents (by aspiration) 3. upper small intestine biopsies 4. Fecal antigen detection by enzyme immunoassays (EIA) 5. enzyme linked immunosorbent assay (ELISA) 6. Direct fluorescence 7. Giardia Western immunoblotting 8. RT-PCR Life cycle notes epidemiology Symptoms/treatment Cyst enters the stomach → gastric acid stimulates cysts to excyst in the duodenum → trophozoites become established and multiply (8 hrs) by longitudinal binary fission → feed by attaching their sucking disks to the mucosa of the duodenum Found in lakes, streams, and other water sources Only known pathogenic intestinal flagellate Major cause of parasitic diarrheal outbreaks in the US Asymptomatic carrier state – asymptomatic Trophozoites may also infect the common bile duct and gallbladder Cysts are viable for 3 months in water Trophozoites disintegrate quickly when entered the outside environment Cysts – resistant to routine chlorination Filtration and chemical treatment should be done for drinking water Transmitted by eating contaminated fruits/veggies Person-to-person contact through oralanal sexual practices or fecal-oral route High risk of those in day care centers, people living in poor sanitary conditions and those who practice unprotected sex Giardiasis (traveler’s diarrhea) - Mild diarrhea, abdominal cramps, anorexia, and flatulence to tenderness of the epigastric region, steatorrhea, and malabsorption syndrome - Light colored stools w/ high fat content - Fat-soluble vitamin deficiencies, hypoproteinemia with hypogammaglobulinemia and structural changes in intestinal villi Incubation period: 10-36 days TREATMENT: 1. Metronidazole (Flagyl) 2. Tinidazole (Tindamax) 3. Nitazoxanide (Alinia) Prevention and control Proper water treatment (chemical therapy and filtration) Good personal hygiene Proper cleaning Cooking of food Avoidance of unprotected oralanal sex Double-strength saturated iodine solution may be added to potentially contaminated water Flagellate Chilomastix mesnili Morphology Lab diagnosis Epidemiology Trophozoites – freshly passed liquid stools Found in warm climates Cysts – formed stools Both – semiformed consistency Stain: iodine wet prep Transmission – ingestion (hand-tomouth contamination or contaminated food or drink) Symptoms/treatment Prevention and control Asymptomatic Good personal hygiene Good public sanitation Flagellate Morphology Dientamoeba fragilis Lab diagnosis Life cycle notes epidemiology Symptoms/treatment Stool samples Reside in the mucosal crypts of the large intestine Exact mode of transmission is unknown Asymptomatic carrier state: Asymptomatic Multiple samples as amount of parasite shedding may vary form day-to-day Has the ability to blend in well with the background material RT-PCR – most sensitive of diagnostic methods No known cyst stage Rarely known to ingest RBC Those who are at risk of contracting: 1. Children 2. Homosexual men 3. Those living in semicommunal groups 4. People who are institutionalized Symptomatic: diarrhea, abdominal pain, bloody/mucoid stools, flatulence, nausea, vomiting, weight loss, fatigue or weakness, constipation, low-grade eosinophilia, and pruritus TREATMENT: Iodoquinol or Paromomycin (humatin) Prevention and control Good personal and public sanitary conditions Avoidance of unprotected homosexual practices Eradication of the helminth eggs Flagellate Morphology Trichomonas hominis Lab diagnosis Life cycle notes Symptoms/treatment Stool samples Found in warm and temperate Asymptomatic Children appear to contract this more often than adults No treatment indication Prevention and control Improved personal and public sanitary practices Transmission: ingesting trophozoites (mostly contaminated milk) and fecaloral transmission No known cyst stage Flagellate Enteromonas hominis Morphology Lab diagnosis Life cycle notes Symptoms/treatment Stool samples Found in warm and temperate Asymptomatic Transmission: ingesting of infected cysts No treatment indication Prevention and control Proper personal hygiene Flagellate Retortamonas intestinalis Morphology Lab diagnosis Life cycle notes Symptoms/treatment Stool samples Found in warm and temperate Asymptomatic Transmission: ingestion of infected cysts No treatment indication Prevention and control Improved personal and public sanitary practices Flagellate Morphology Trichomonas tenax Lab diagnosis Life cycle notes Symptoms/treatment Mouth scrapings (tartar between the teeth and gingival margin) trophozoites survive in the body as mouth scavengers that feed primarily on local microorganisms. Invades respiratory tract (but affects those who have underlying disease) multiply by longitudinal binary fission. No notable symptoms unable to survive the digestive process Flagellate Morphology Trichomonas vaginalis No known cyst stage No treatment indication Lab diagnosis Life cycle notes Symptoms/treatment spun urine, vaginal discharges, urethral dis charges, and prostatic secretions. reside on the mucosal surface of the vagina in infected women Persistent Urethritis: enlarged tender prostate, dysuria, nocturia, and epididymitis. Thin, white urethral discharge Saline wet preparations Persistent Vaginitis: a foul-smelling, greenish-yellow liquid vaginal discharge after an incubation period of 4 to 28 days. Burning, itching, and chafing, thrive in a slightly alkaline Urethral or slightly acidic pH involvement, dysuria, and increased environment, such as frequency of urination that commonly seen in an Infant infections: recovered from infants unhealthy vagina. suffering from both respiratory infection and conjunctivitis. Male: prostate gland region and the epithelium Metronidazole (Flagyl) of the urethra Other tests: phase contrast microscopy, Papanicolaou (Pap) smears, fluorescent stains, monoclonal antibody assays, enzyme immunoassays, and cultures. InPouch TV culture system multiply by longitudinal binary fission and feed on local bacteria and leukocytes Prevention and control Good oral hygiene Prevention and control Avoid unprotected sex avoidance of sharing douche equipment and communal bathing, and close contact with potentially infective underclothing, toilet articles, damp towels, and wet sponges HEMOFLAGELLATES • Members of the clinically significant group of parasites located in blood and tissue that move by means of flagella, known as the hemoflagellates, belong to the genera Leishmania and Trypanosoma. body length, forms into a free flagellum at the anterior end of the epimastigote. FOUR MORPHOLOGIC FORMS: Amastigotes - measures 5 by 3 μm in size. contains a nucleus, a basal body structure (called a blepharoplast), and a small parabasal body. Kinetoplast is an umbrella term often used to refer to the blepharoplast and small parabasal body. Promastigotes - measures 9 to 15 μm in length. large single nucleus is located in or near the center of the long slender body. The kinetoplast is located in the anterior end of the organism. A single free flagellum extends anteriorly from the axoneme. Epimastigotes – measures approximately 9 to 15 μm in length. Body is slightly wider than promastigote. The single nucleus is located in the posterior end of the organism. The kinetoplast is located anterior to the nucleus. An undulating membrane, measuring half the • Leishmaniasis is a general term used to describe diseases caused by the hemoflagellate genus Leishmania. • Diseases caused by Leishmania spp. (e.g., Baghdad boils, bay sore, chiclero ulcer, dum dum fever, espundia, forest yaws, kala-azar, oriental sore, pianbois, and uta). Trypanosomiasis is a general term used to refer to human diseases caused by hemoflagellates of the genus Trypanosoma. These diseases have been well documented through the ages. Ancient papyri discussed the disease from veterinary and human perspectives. Morphological forms AMASTIGOTES PROMASTIGOTES Morphology EPIMASTIGOTE TRYPOMASTIGOTES Lab diagnosis • Blood, lymph node and ulcer aspirations, tissue biopsies, bone marrow, and cerebrospinal fluid (CSF) are the specimens of choice for diagnosing the hemoflagellate morphologic forms. In addition, serologic and molecular tests are also available for confirming the presence of these organisms. Clinical symptoms • symptoms associated with hemoflagellate infections range from a small red papule at the infection site, with intense itching, secondary bacterial infections, fever, and diarrhea, to kidney involvement, mental retardation, a comatose state, and death. In some cases, the initial skin lesions spontaneously heal, whereas in others they may remain dormant for months or even years. Hemoflagellate Leishmania braziliensis complex Common associated disease and condition names: Mucocutaneous leishmaniasis, chiclero ulcer, espundia, forest yaws, pian bois, uta. Morphology Lab diagnosis Life cycle notes Epidemiology Symptoms / treatment Prevention - specimen of choice for identifying the amastigotes of L. braziliensis complex is a biopsy of the infected ulcer. - Sandflies of the genera Lutzomyia and Psychodopygus are responsible for transmitting the promastigotes of the species of the L. braziliensis complex into unsuspecting humans via a blood meal, resulting in a skin bite. - diagnostic stage for the species of the L. braziliensis complex is the amastigote. - the amastigote serves as the infective stage for the sandfly. - ingestion, during a blood meal of an infected human, the amastigotes transform back into promastigotes - composed of L. braziliensis (found from Mexico to Argentina), L. panamensis (found in Panama and Colombia), L. peruviana (found in the Peruvian Andes), and L. guyanensis (found in Guiana, parts of Brazil, and Venezuela). Mucocutaneous Leishmaniasis. - Microscopic examination of the Giemsa-stained preparations – amastigote - Promastigotes may be present when the sample is collected immediately after introduction into the patient. - infected material, which often demonstrates the promastigote stage, and serologic testing. - Enzyme analysis - also be found in Ecuador, Bolivia, paraguay, and other Central and South American countries, particularly in the rain forest regions, where chicle sap for chewing gum is Harvested. - Transmission is generally through the bite of the Lutzomyia or Psychodopygus - occur within a few weeks to months after transmission into a previously uninfected human. Large ulcers in the oral or nasal mucosa areas (mucocutaneous) - A cutaneous (meaning affecting or relating to the skin) lesion may heal on its own. Untreated cases – destruction of nasal septum - lips, nose, and other surrounding soft parts may also be affected in these infections. Edema and secondary bacterial infections, combined with numerous mucosal lesions, may cause disfigurement of the patient’s face. - Public awareness through education programs in endemic areas and exercising personal protection against contact with sandflies (e.g., protective clothing, repellents, screening) - prompt treatment and eradication of infected ulcers, and control of the sandfly population and reservoir Hosts. - Work to produce a vaccine against members of Leishmania donovani complex Common associated disease and condition names: Visceral leishmaniasis, kala-azar, dum dum fever. - Molecular techniques - Kinetoplast DNA / scizodeme analysis - Nuclear DNA hybridization - zymodeme analysis in the fly midgut. These promastigotes multiply and the resulting developed forms eventually migrate into the salivary gland of the fly, where they are ready to be transferred to a new human during a blood meal. - Montenegro skin test (similar to tuberculin skin test) - identical to that - Giemsa-stained slides of blood, bone marrow, lymph node aspirates, and biopsies of the infected areas are better choices for demonstrating the diagnostic amastigote forms. - Serologic of L. braziliensis, with only two exceptions. First, the specific sandfly species responsible for L. donovani transmission vary with each of the three subspecies. - Second, L. donovani primarily affects the visceral tissue of the infected human. - Death is usually attributed to a secondary bacterial infection. Treatment: - sodium stibogluconate (Pentosam). - liposomal amphotericin B (Ambisome) and oral antifungal drugs such as fluconazole (Diflucan), ketoconazole(Nizoral) and itraconazole (Sporonox). - composed of L. donovani (found in India, Pakistan, Thailand, parts of Africa, and the Peoples Republic of China), L. infantum (found in the Mediterranean area, Europe, Africa, the Near East, and parts of the former Soviet Union), and L. chagasi (found in Central and South America). L. donovani and - Visceral Leishmaniasis. - also known as kala-azar( black fever) or dum dum fever, often present with a nondescript abdominal illness and hepatosplenomegaly (enlargement of the spleen and liver). - may resemble malaria or typhoid fever with the development of fever and chills. symptoms is gradual and follows an incubation the L. braziliensis complex and other Leishmania spp. is ongoing, with some vaccines for animals (dogs) presently in experimental trials. - Protection against sandflies by repellents, protective clothing, and screening are essential measures to reduce future L. donovani complex infections. Prompt treatment of human infections, as well as control of the sandfly population and reservoir hosts, will also testing is available using IFA (indirect fluorescent antibody), ELISA (enzyme-linked immunosorbent assay), and DAT (direct agglutination test). - person to person via blood transfusions. -leishmaniasis transmission arose during and following the Gulf War. L. infantum are known to be endemic in areas of the Middle East, including Yemen, Oman, Kuwait, Iraq, Saudi Arabia, the United Arab Emirates, and Bahrain. period ranging from 2 weeks to 18 months. - Diarrhea, as well as anemia, may often be present. weight loss and emaciation, tend to occur following parasitic invasion of the liver and spleen. a rare papule, which mostly occurs at the bite site, skin lesions are absent. Advanced stages of disease result in kidney damage (e.g., glomerulonephritis, inflammation of the glomeruli of the kidney) and granulomatous areas of skin. A characteristic darkening of the skin may be noted. - Chronic cases leads to death in 1 or 2 years. acute disease debilitates the patient and becomes lethal in a matter of weeks. TREATMENT: - Liposomal amphotericin B (Ambisome), Sodium stibogluconate (Pentosam) - use of gamma interferon combined with pentavalent antimony. help halt the spread of disease. Leishmania Mexicana complex Common associated disease and condition names: New World cutaneous leishmaniasis, chiclero ulcer, bay sore. - by demonstrating the amastigote form in Giemsa-stained preparations of lesion biopsy material. - Culture on NNN medium demonstrates the promastigote stage of these organisms. - Serologic testing using monoclonal antibodies. Schizodeme analysis, zymodeme analysis, and nuclear DNA hybridization are - The life cycle of the members of the L. mexicana complex is identical to that of L. braziliensis. The primary vectors are sandfly species of the genus Lutzomyia. - composed of L. mexicana (found in Belize, Guatemala, and the Yucatan peninsula), L. pifanoi (found in the Amazon River basin and parts of Brazil and Venezuela), L. amazonensis (found in the Amazon basin of Brazil), L. venezuelensis (found in the forested areas of Venezuela), and L. garnhami (found in the Venezuelan Andes). Members of this complex are often - allopurinol (AIDS PATIENT) - that HIV-infected persons receive secondary prophylaxis as part of their treatment plan. - visceral leishmaniasis which include a combination of paramomycin and miltefosine. Neither of these drugs is available in the United States at this time. - New World Cutaneous Leishmaniasis. Also known as bay sore and chiclero ulcer, cutaneous leishmaniasis usually characterized by a single pus-containing ulcer, which is generally selfhealing. - 40% of infections affect the ear and can cause serious damage to the surrounding cartilage. of anergic (the inability of an individual to mount an adequate immune response) and hypersensitivity immunologic responses, spontaneous healing of the ulcers does not occur. - Protection against sandflies by repellents, protective clothing, and screening are essential measures to reduce future L. mexicana complex infections. Prompt treatment of human infections, as well as control of the sandfly and reservoir host populations, will also help halt the available on a research basis. transmitted by the bite of a Lutzomyia sandfly, with forest rodents serving as the reservoir host. - infections with L. pifanoi, the initial lesion appears, ulcerates or disappears and, after a period of months to years, appears in local and distant areas from the bite site with lepromatousappearing lesions. L. amazonensis infections have been known to progress to an incurable diffuse cutaneous form of the disease. cutaneous leishmaniasis usually occurs when the patient is anergic. TREATMENT: - Pentavalent antimonials, such as sodium stibogluconate (Pentosam), Antimony combined with pentoxifylline taken orally three times a day for 30 days has been shown to be superior to antimony alone. Amphotericin B and liposomal amphotericin B (Ambisome) have also proven to be effective. spread of disease. Leishmania tropica complex Common associated disease and condition names: Old World cutaneous leishmaniasis, oriental sores, Delhi boils, Baghdad boils, dry or urban cutaneous leishmaniasis. - With the exception of the examination of specific sandfly Giemsa-stained slides (reveal species amastigotes). and the area of the body most - Culture of the affected, the life under of the ulcer cycle of L. tropica tissue may also complex is revels promastigote. basically identical to that of L. - Serologic tests, braziliensis. All such as IFA testing, three of the L. are available. Schizodeme analysis, tropica zymodeme analysis, subspecies are and nuclear DNA transmitted by hybridization are the Phlebotomus also available on a sandfly. L. tropica research basis. complex primarily attacks the human lymphoid tissue of the skin. - Microscopic - protection by the use of L. tropica (found in Leishmaniasis. protective the Mediterranean region, Middle East, clothing, Old World leishmaniasis, Armenia, Caspian repellents, and oriental sore, and Baghdad region, Afghaniscreening are or Delhi boil, cutaneous stan, India, and leishmaniasis is characterized essential to Kenya), L. aethiopica prevent future by one or more ulcers (found in the containing pus that generally L. tropica highlands of self-heal. complex Ethiopia, Kenya, and infections. In Southern - develop a small red papule, addition, the Yemen), and L. major located at the bite site, prompt (found in the desert which is typically 2 cm or treatment and region of larger in diameter and may Turkmenistan, eradication of cause intense itching. Uzbekistan, and infected ulcers Kazakhstan, are crucial to - On occasion, because of Northern Africa, the halt disease anergic and hypersensitivity Sahara, Iran, Syria, transmission. A immunologic responses, Israel, and Jordan). vaccine has spontaneous healing of the Members of this ulcers does not occur. DCL been complex are often occurs especially on the developed and transmitted by the limbs and face when an the preliminary bite of the immune response fails to results are Phlebotomus take place. promising; sandfly, but the reservoir hosts for however, the TREATMENT: each of the three clinical trials for - effective treatment of L. members of this this vaccine are tropica complex is sodium complex differ still ongoing. stibogluconate (Pentosam). - composed of - Old World Cutaneous The use of steroids, application of heat to the Trypanosoma brucei gambiense Common associated disease and condition names: West African sleeping sickness, Gambian trypanosomiasis. T. brucei gambiense is found in the tropical areas of western and central Africa. Commonly called West African sleeping sickness or Gambian trypanosomiasis. acquired through blood transfusion, organ transplantation, and congenital transmission (from pregnant mother to fetus). - Blood, lymph node - infected with T.b. aspirations, and CSF are the specimens of choice for diagnosing T.b. gambiense. - Giemsa-stained slides of blood and lymph node aspirations from infected patients reveal the typical trypomastigote morphologic forms. - CSF—microscopic examination of the sediment for trypomastigotes, detection of the presence of immunoglobulin M (IgM), and detection of the gambiense following the injection of trypomastigotes by the tsetse fly during its blood meal. The entering trypomastigotes migrate through the bloodstream and into the lymphatic system, multiplying by binary fission. - invasion of the CNS may occur. The trypomastigotes are transmitted back to the tsetse fly vector when it feeds on an - found in tropical West Africa and Central Africa, especially in shaded areas along stream banks where the tsetse fly vector breeds. The two species of tsetse flies responsible for the transmission of T.b. gambiense are Glossina palpalis and Glossina tachinoides. There are no known animal reservoir hosts. infected lesions, meglumine antimonate (Glucantime), pentamidine, and oral ketoconazole may be indicated for treating L. tropica complex infections. Paromomycin ointment may also be given to aid in healing. - West African (Gambian) Sleeping Sickness. - West African sleeping sickness begin to occur after an asymptomatic incubation period of a few days to several weeks. - painful chancre (ulcer), surrounded by a white halo at the bite site. Fever, malaise, headache, generalized weakness, and anorexia are often experienced when the trypomastigotes settle into the lymphatic system. lymph node enlargement (lymphadenopathy) may be apparent during this time. - Winterbottom’s sign refers to the enlargement of the cervical lymph nodes. in reference to this trypanosomal disease. Other - destroying their breeding areas via chemical treatment and clearing of brush. Proper protective clothing, repellents, and screening, as well as prompt treatment of infected persons, presence of proteins. Infected patients typically have high levels of both IgM and proteins in their CSF. In addition, serum IgM testing may be indicated. The presence of IgM in serum and/or CSF is generally considered diagnostic. A number of serologic tests are also available. infected human. Once ingested by the tsetse fly, the trypomastigotes continue to multiply and eventually migrate back to the salivary gland, converting into epimastigotes along the way. Once in the salivary gland, the epimastigotes transform back into trypomastigotes, thus completing the cycle. symptoms that may be seen during the glandular phase of the disease include erythematous (red) rash, pruritis, localized edema (swelling), and Kerandel’s sign (a delayed sensation to pain). In patients in whom the central nervous system (CNS) becomes involved, mental retardation, tremors, meningoencephalitis, somnolence (excessive sleepiness), and character changes may develop. In the final stage of disease, the patient slips into a coma and death occurs. TREATMENT: - melarsoprol, suramin, pentamidine, and eflornithine. The treatment of choice is situation dependent and is dictated by a number of factors, including patient age (adult, child), stage of disease, and whether the patient is pregnant at the time. Trypanosoma brucei rhodesiense Common associated disease and condition names: East African sleeping sickness, Rhodesian trypanosomiasis. Trypanosoma brucei rhodesiense is found in eastern and central Africa. Commonly called East African sleeping sickness or Rhodesian trypanosomiasis, the disease course for the illness caused by this organism is much more aggressive than that of its West African counterpart. - detection of the typical T.b. rhodesiense trypomastigotes are blood slides stained with Giemsa and microscopic examination of CSF sediment. Protein and IgM studies on CSF may also be performed. As with T.b. gambiense, the presence of IgM in the CSF is diagnostic for T.b. rhodesiense. - only difference in the life cycles of T.b. rhodesiense and T.b. gambiense are the species of tsetse fly vector. The two primary species of tsetse fly vectors responsible for transmitting T.b. rhodesiense are Glossina morsitans and Glossina pallidipes. Additional species noted for attacking game animals may also transmit this organism. - found in East and - East African (Rhodesian) Central Africa, especially in brush areas. Cattle and sheep, as well as wild game animals, are known reservoir hosts of this organism. Sleeping Sickness. - much more virulent organism than T.b. gambiense. Following a short incubation period, patients suffering from acute East African sleeping sickness experience fever, myalgia, and rigors. Winterbottom’s sign may or not be present. Lymphadenopathy is absent. Rapid weight loss is common and the CNS becomes involved early in the disease course. In addition, mental disturbance, lethargy, and anorexia may also be present. - Death, in part caused by subsequent kidney damage (glomerulonephritis) and myocarditis (inflammation of the heart), usually occurs within 9 to 12 months in untreated patients. TREATMENT: Identical to T.b gambiense - extremely early treatment is crucial to halt further transmission of the disease. Additional measures include prompt medical treatment of infected domestic animals, as well as protective clothing, screening, and repellents. - breeding may occur wherever brush is abundant, primarily away from water sources, complicates prevention and control efforts. The clearing of brush areas and control of the tsetse fly population may reduce the risk of future disease transmission. Trypanosoma cruzi Common associated disease and condition names: Chagas’ disease, American trypanosomiasis. Trypanosoma cruzi is found in southern portions of the United States, Mexico, and Central and South America. Commonly referred to as Chagas’ disease or American trypanosomiasis, the disease course for this illness often presents itself with cardiac and gastrointestinal distress. endemic areas of South America is known as xenodiagnosis. - Giemsa-stained blood slides are the specimen of choice for detection of the typical T. cruzi trypomastigotes. Epimastigotes may rarely be seen in the circulating blood; however, this form is primarily found only in the arthropod vector. Lymph node biopsy Giemsastained slides, as well as blood culture, may reveal the typical amastigotes. - most frequently transferred to a human host when a reduviid bug vector defecates infective trypomastigotes near the site of its blood meal. - the bite produces an itching sensation in the host. As the host scratches the bite area, the trypomastigotes conveniently gain entry into the host by literally being rubbed into the bite wound. - complement fixation (CF), DAT, and indirect immunofluorescence - blood (IIF), polymerase transfusions, chain reaction (PCR) sexual intercourse, - found primarily in South and Central America and only rarely in North America. The highest known prevalence of disease is in Brazil. - Although first isolated in a Panstrongylus megistus, there are additional reduviid bug species that may serve as vectors. Also known as the kissing bug, conenose bug, and triatomid bug, the reduviid bug nests in human homes that are open in design. Although there are a number of known - Chagas’ Disease. - asymptomatic, chronic, or acute in nature. The most common initial symptom is the development of an erythematous nodule, known as a chagoma, at the site of infection produced by the proliferation of the T. cruzi organisms. - most frequently located on the face. Edema as well as a rash around the eyes and face may subsequently occur. The painful chagoma may last 2 to 3 months before subsiding. Patients who contract T. cruzi through the ocular mucosa develop a characteristic conjunctivitis and unilateral edema of the eyelids, a condition known as Romaña’s sign. - eradication of reduviid bug nests and the construction of homes without open design are crucial measures necessary to help alleviate the future spread of the disease. DDT has proved to be useful, not only to control the reduviid population but also to decrease the incidence of malaria. - bug infested homes. and ELISA testing methods transplacental transmission, and entry through the mucous membranes when the bug bite is near the eye or mouth. - trypomastigotes invade surrounding cells, where they transform into amastigotes. The amastigotes proceed to multiply, destroy the host cells, and then convert back into trypomastigotes. The resulting trypomastigotes migrate through the blood, penetrate additional cells in the body, and transform back into amastigotes, and the replication and destruction cycle repeats. A number of areas in mammalian hosts, dogs and cats are of particular importance as reservoir hosts in Brazil. - Chronic Chagas’ disease may occur after the initial diagnosis of an acute disease or years to decades after being initially asymptomatic. - myocarditis, enlargement of the colon (sometimes referred to as megacolon) and esophagus (sometimes referred to as megaesophagus), and hepatosplenomegaly. In addition, CNS involvement, cardiomegaly (enlargement of the heart), and electrocardiographic changes may be seen. Complete blockage of the heart, as well as brain damage, may result, causing sudden death. - acute Chagas’ disease typically experience fever, chills, fatigue, myalgia, and malaise. An attack of acute infection may result in one of the following scenarios: (1) recovery; (2) transition to the chronic stage of disease; or (3) death, which usually occurs a few weeks after the attack. Educational programs designed to inform people, especially in endemic areas, of the disease, its transmission, and possible reservoir hosts. Developing vaccine would help the body may become infected, including the heart muscle, liver, and brain. - trypomastigotes are transmitted back to the reduviid bug when it feeds, via a blood meal, on an infected human. On ingestion, the trypomastigotes transform into epimastigotes in the midgut. Multiplication of the epimastigotes produces thousands of additional parasites that convert back into trypomastigotes when they reach the hindgut. These trypomastigotes are then passed with the feces when the bug The frequency and form of Chagas’ disease seen in small children versus older children and adults vary. In general, Chagas’ disease is most commonly seen in children younger than 5 years. TREATMENT: - nifurtimox (Lampit). Other medications include benznidazole, allopurinol, and the antifungal agent ketoconazole. Trypanosoma rangeli Common associated disease and condition names: None known. Trypanosoma rangeli is found in many of the same geographic regions as T. cruzi. There are presently no common names known for disease caused by this organism. Infections are generally asymptomatic and tend to show no pathologic changes or signs of disease. - Giemsa-stained blood slides are the specimen of choice for the detection of the typical T. rangeli trypomastigotes. T. rangeli trypomastigotes can be seen in the peripheral blood throughout the course of the illness. It can also be diagnosed by xenodiagnosis and serologic testing methods. PCR-based methods are also available. defecates near the site of its next blood meal, and thus the cycle begins again. - similar to that of T. cruzi. The vector responsible for transmitting T. rangeli is the reduviid bug, Rhodius prolixus. - This vector actually transmits the parasitic infection via its saliva. T. rangeli can be viewed in the blood throughout the course of the infection. - commonly found in the same geographic areas as T. cruzi—regions of South and Central America, particularly in the areas surrounding Brazil, Venezuela, Colombia, Panama, El Salvador, Costa Rica, Honduras, and Guatemala. Its vector, Rhodius prolixus, is attracted to the same open house design as other reduviid bug species. It has numerous reservoir hosts such as monkeys, raccoons, dogs, cats, armadillos, and rodents. - Patients infected by T. rangeli are generally asymptomatic and demonstrate no evidence of illness. It is generally thought to be a benign infection. TREATMENT: - Nifurtimox and benzimidazole - Prevention and control measures for T. rangeli are the same as those for T. cruzi. - 6.1 BACKGROUND - Phylum Apicomplexa - Class Aconoidasida - Order haemosporida - Plasmodium 10 species infect humans 1. P. vivax 2. P. ovale 3. P. malariae 4. P. falciparum 5. P. knowlesi - Babesia 4 species infect humans 1. B. microti 2. B. divergens - FORMS: 1. Ring form (early trophozoite) - Ring like appearance - Consist of blue cytoplasmic circle connected to a red chromatin dot - Has a vacuole 2. Developing trophozoite - Remnants of the cytoplasmic circle and chromatin dot - Pigment (brown) is visible 3. Immature schizont - Visible cytoplasmic material - Active in chromatin replication - Pigment granules (brown) is visible 4. Mature schizont - Fully developed stage of merozoites - Cytoplasmic material is not visible 5. Microgametocyte - Round in shape (except P. falciparum) - Pink to purple large diffuse chromatin mass surrounded by colorless halo 6. Macrogametocyte - Round to oval shape (except P. falciparum) - Compact chromatin mass surrounded by cytoplasmic material - LIFE CYCLE NOTES - ANOPHELES responsible for the transmission of malaria to humans via a blood meal - Transfers the infective stage of the parasite known as sporozoites from its salivary gland into the human bite wound - Schizogony occurs (asexual multiplication) - EXOERYTHROCYTIC CYCLE (reproduction outside RBC) occurs from 8-25 days - HYPNOZOITES dormant Plasmodium-infected liver cells - GAMETOCYTES transmission of the parasite back into the vector occurs when the mosquito ingests mature male (micro) and female (macro) sex cells - ZYGOTE fertilized cell that was formed when the male and female gametocytes unite in the (aka ookinete) OOCYST encysted and mature zygote 6.2 LABORATORY DIAGNOSIS - Giesma-stained peripheral blood films specimen of choice for the laboratory diagnosis - Serologic tests - PCR tests - Thick blood smears for screening - Thin blood smears for differentiating - Blood films to be studied under oil immersion - The greatest number of parasites is present in the blood in between characteristic bouts of fever and chills resulting from the release of merozoites and toxic waste products from infected RBCs aka PAROXYSMS - Blood collection every 6-12 hours for up to 48 hrs before considering a patient to be free of Plasmodium spp. Parasites ANOPHELES SPP. - Carrier of the plasmodium parasite BABESIA SPECIES 6.3 PATHOGENESIS AND CLINICAL SYMPTOMS - PAROXYSMS - once the erythrocytic phase is initiated and large numbers of rupturing RBCs simultaneously occur, the resulting merozoites and toxic waste byproducts in the blood system produce the first clinical symptom - RIGOR a paroxysm is characterized by chills (lasts about 10-15 mins) - RECRUDESCENCE occurs when patients become reinfected with rupturing with rupturing hypnozoites months to years after the initial infection - OTHER SYMPTOMS: a. Headache b. Lethargy c. Anorexia d. Ischemia e. Nausea f. Vomiting g. Diarrhea h. Anemia i. CNS involvement j. Nephrotic syndrome - May mimic a number of other diseases a. Meningitis b. Pneumonia c. Gastroenteritis d. Encephalitis e. Hepatitis - - - - SEXUAL occurs within its tick ASEXUAL occurs within its host generally transmitted through the bite of an infected tick of the genus Ixodes. The ookinete travels to the salivary glands where sporogony the process of spore and sporozoite production via sexual reproduction takes place, resulting in numerous sporozoites that can be transmitted to a new host LABORATORY DIAGNOSIS - Giemsa-stained peripheral blood films - Wright - Thick and thin blood films - Thin differentiate - All blood films should be studied under oil immersion - serologic tests - PCR techniques PATHOGENESIS AND CLINICAL SYMPTOMS - 1 to 4 weeks prior to the onset of symptoms. - prodrome-like symptoms fever, headache, chills, sweating, arthralgias, myalgias, fatigue, and weakness. - Hepatosplenomegaly and mild to severe hemolytic anemia - Elevated bilirubin and transaminase levels - worse for the splenectomized and immunocompromised patient. B. divergens - EPIDEMIOLOGY - VECTOR Ixodes dammini - Principal reservoir host Peromyscus leucopus - Found in European countries - Transmission vector Ixodes Ricinus - CLINICAL SYMPTOMS - B. divergens tends to be the more severe of the two parasitic infections and is frequently fatal if left untreated. - - - B. microti tends to be rather benign and selflimiting. - Disease with either of these organisms is often more severe for older adult, immunosuppressed, and/or splenectomized patients. TREATMENT - Diminazene and pentamidine, in combination or singly, and pyrimethamine and quinine, in combination or singly PREVENTION AND CONTROL - avoid tick infested areas. - tick must feed for at least 12 hours before it is able to transmit the parasite. - Using insect repellents - eradicating the tick population PARASITE P. vivax Benign MORPHOLOGY LAB DIAGNOSIS LIFE CYCLE NOTES Thin blood films diagnosis Invade young RBCs (as they are pliable) Thick blood films Distortion of the RBCs tertian malaria Vivax malaria Observe during halfway between paroxysms EPIDEMIOLOGY CLINICAL SYMPTOMS / TREATMENT Tropics and BENIGN TERTIAN MALARIA subtropics - 10-17-day incubation period postexposure Temperate - Nausea, vomiting, headache, regions muscle pains and photophobia - Paroxysms every 48 hours - When chronic may damage brain, liver, and kidney TREATMENT - quinine, quinidine, chloroquine, amodiaquine, primaquine, pyrimethamine, sulfadoxine, dapsone, mefloquine, tetracycline, doxycycline, halofantrine, atovaquone, proguanil, ginghaosu, artemisinin, artemether, artesunate, pyronaridine, Fenozan B07, trioxanes, nonane endoperoxides, azithromycin, and WRZ38605. PREVENTION AND CONTROL Personal protection netting, screening, protecting clothing and repellents Prophylactic treatment Avoidance of sharing IV needles Thorough screening of donor blood PARASITE MORPHOLOGY P. ovale Benign LAB DIAGNOSIS Thin blood films speciate tertian malaria Thick blood films presence Mature schizont choice for examination Note may develop ragged cell walls in response to the growing parasite LIFE CYCLE NOTES Invade young RBCs (as they are pliable) EPIDEMIOLOGY CLINICAL SYMPTOMS / TREATMENT Tropical Africa Asia South America BENIGN TERTIAN MALARIA AND OVALE MALARIA - paroxysm (every 48 hours) - relapses caused by reactivation of hypnozoites - infections that last approximately 1 year TREATMENT - quinine, quinidine, chloroquine, amodiaquine, primaquine, pyrimethamine, sulfadoxine, dapsone, mefloquine, tetracycline, doxycycline, halofantrine, atovaquone, proguanil, ginghaosu, artemisinin, artemether, artesunate, pyronaridine, Fenozan B07, trioxanes, nonane endoperoxides, azithromycin, and WRZ38605. PREVENTION AND CONTROL Personal protection netting, screening, protecting clothing and repellents Prophylactic treatment Avoidance of sharing IV needles Thorough screening of donor blood PARASITE MORPHOLOGY LAB DIAGNOSIS LIFE CYCLE NOTES EPIDEMIOLOGY CLINICAL SYMPTOMS / TREATMENT P. malariae See table below Thin and thick blood films w/ Giesma stains Invade young RBCs (as they are pliable) Subtropic and temperate Quartan malaria Malarial malaria Take note multiplies within the confines of mature RBCs. Does not contain Ring stage not commonly seen Usually seen developing trophozoite, immature and mature schizonts QUARTAN MALARIA - incubation of 18-40 days followed by flu-like symptoms - cyclic paroxysms every 72 hrs - no known relapses because dormant hypnozoites are not associated with P. malariae infections TREATMENT - quinine, quinidine, chloroquine, amodiaquine, primaquine, pyrimethamine, sulfadoxine, dapsone, mefloquine, tetracycline, doxycycline, halofantrine, atovaquone, proguanil, ginghaosu, artemisinin, artemether, artesunate, pyronaridine, Fenozan B07, trioxanes, nonane endoperoxides, azithromycin, and WRZ38605. PREVENTION AND CONTROL Personal protection netting, screening, protecting clothing and repellents Prophylactic treatment Avoidance of sharing IV needles Thorough screening of donor blood Control mosquito breeding areas PARASITE MORPHOLOGY LAB DIAGNOSIS P. falciparum Thick blood films screening Black water Thin blood films speciation fever LIFE CYCLE NOTES Invade RBC at any age Infect up to 50% of the RBC population Peripheral blood films reveal ring forms Schizogony and gametocyte occur in capillaries and blood sinuses Infect RBCs but do not appear enlarged Occur in the or distorted warmer months of late summer and Recrudescence may early autumn occur and is fatal Aka aestivoautumnal malaria Malignant tertian malaria Aestivoautumnal malaria fnblertian malaria falciparum malaria Infects RBCs at any age EPIDEMIOLOGY CLINICAL SYMPTOMS / TREATMENT Tropical and subtropical regions BLACK WATER FEVER AND MALIGNANT TERTIAN MALARIA - incubation of 7-10 days followed by flu-like symptoms - cyclic paroxysms every 36-48 hrs - mimic those seen in malignant tertian malaria - most deadly form if malaria in untreated patients - Kidney involvement, known as black water fever, usually results in marked hemoglobinuria (the presence of hemoglobin in the urine) caused by P. falciparuminduced red cell destruction. - Acute renal failure, tubular necrosis, nephrotic syndrome and death - Coma death - Abdominal pain, vomiting of bile, rapid dehydration and sever diarrhea TREATMENT - quinine, quinidine, chloroquine, amodiaquine, primaquine, pyrimethamine, sulfadoxine, dapsone, mefloquine, tetracycline, doxycycline, halofantrine, atovaquone, proguanil, ginghaosu, artemisinin, artemether, artesunate, pyronaridine, Fenozan B07, trioxanes, nonane endoperoxides, azithromycin, and WRZ38605. - Malaria is treated first, then secondary complicating health problems PREVENTION AND CONTROL Personal protection netting, screening, protecting clothing and repellents Prophylactic treatment Avoidance of sharing IV needles Thorough screening of donor blood Control mosquito breeding areas Vaccine PARASITE LAB DIAGNOSIS LIFE CYCLE NOTES CLINICAL SYMPTOMS / TREATMENT PREVENTION AND CONTROL P. knowlesi DNA extraction and nested-PCR examination of samples have been known to reveal the differences between the two Plasmodium species resemble P. falciparum CLINICAL FEATURES - Respiratory distress Acute renal or multi-organ failure - Shock Personal protection netting, screening, protecting clothing and repellents Giesma-stained peripheral blood films specimen of choice Wright stain (thick for screening; thin for differentiate) PCR TREATMENT - No complications a. quinine, b. chloroquine c. artemetherlumefantrine - severe a. Inraveous quinine b. artesunate c. combination of chloroquine-primiquine Prophylactic treatment Avoidance of sharing IV needles Thorough screening of donor blood Control mosquito breeding areas Vaccine CLASSIFICATION: - Ciliates - parasites that move by means of hairlike cytoplasmic extensions called cilia, contains one human pathogen known as Balantidium coli - Select Sporozoa – (excluding Plasmodium and Babesis spp.) which are intestinal and tissue-dwelling in nature, belong to the subclass Coccidia, a group of protozoal parasites in which asexual replication occurs outside a human host and sexual replication occurs inside a humanhost, and are often referred to as coccidian protozoans. - Blastocystis hominis (Fig. 7-3), initially considered as a yeast, makes up the third group and is now classified as a Protozoa. This organism is the sole member of the class Blastocystea. - Pneumocystis jiroveci (formerly known as Pneumocystis carinii) is the only member of the fourth group (Fig. 7-4). This organism was traditionally included with the Protozoa, even though it has been recently reclassified as a fungus. T. gondii (cont.) - TOXOPLASMOSIS: GENERAL SYMPTOMS a. Mild, mimic symptoms of infectious mononucleosis b. Fatigue, lymphadenitis, chills, fever, headache and myalgia c. Maculopapular rash, encephalomyelitis, myocarditis, hepatitis d. Retinochoroiditis w/ subsequent blindness - CONGENITAL TOXOPLASMOSIS - Factors that affect its degree of severity: 1. Antibody protection from the mother 2. Age of the fetus at the time of infection - Subsequent retinochoroiditis (years after initial infection) - CHILD: hydrocephaly, microencephaly, intracerebral calcification, chorioretinitis, convulsions, psychomotor disturbances - Result: mental retardation, severe visual impairment, or blindness - TOXOPLASMOSIS IN IMMUNOCOMPROMISED PATIENTS: - Patients immunosuppressed because of organ transplantation or the presence of neoplastic disease, such as Hodgkin’s lymphoma, have long been known to contract toxoplasmosis as an opportunistic infection - Screen potential donor units for toxoplasmosis prior to transfusion - CEREBRAL TOXOPLASMOSIS IN AIDS PATIENTS - toxoplasmic encephalitis, CNS involvement - early symptoms: headache, fever, altered mental status (inc. confusion), lethargy - developed to: subsequent focal neurologic deficits, brain lesions, convulsions - OTHER INFORMATION: - do not spread into other organs of the body but rather stay confined within the CNS - diagnostic: rise in spinal fluid IgG antibody levels (demonstration of tachyzoites in CSF) - Most infected patients do not have serum levels of IgM antibodies. - The lack of serum IgM coupled with the lack of change in serum IgG levels in these patients suggests that their infections occurred because of a reactivation of a chronic latent infection and not because of an acquired primary infection. PARASITE MORPHOLOGY Balantidium coli LAB DIAGNOSIS LIFE CYCLE NOTES EPIDEMIOLOGY CLINICAL SYMPTOMS / TREATMENT PREVENTION AND CONTROL stool specimens initiated on ingestion of infective cysts in contaminated food or water. Human infection is very low ASYMPTOMATIC: no symptoms Personal hygiene Balantidiasis: - Mild colitis, diarrhea, fullblown clinical balantidiasis - Mucosa and submucosa abscesses and ulcers - Secondary bacterial infection - 15 liquid stools (w/ pus, mucus, blood) - CHRONIC: tender colon, anemia, cachexia and occasional diarrhea - May invade liver, lungs, pleura, mesenteric nodes and urogenital tract Proper sanitation Sigmoidoscopy for patients suffering from sigmoid rectal infection Balantidiasis wet preparations and the permanent stain Trophozoite: aka Balantidium, which means “little bag.” excystation in the small intestine transverse binary fission, from which two young trophozoites emerge. Encystation occurs in lumen Rare transmitted by ingesting contaminated food and water by the oralfecal and person-toperson routes. TREATMENT: - oxytetracycline (Terramycin) and iodoquinol. - Metronidazole (Flagyl) Proper precautions when handling and dealing pigs and its feces PARASITE MORPHOLOGY LAB DIAGNOSIS LIFE CYCLE NOTES EPIDEMIOLOGY CLINICAL SYMPTOMS PREVENTION / TREATMENT AND CONTROL Isospora belli Stool samples (immature, partially or fully mature) – Sheather’s sugar flotation procedure Coccidial parasite Rare but has worldwide geographic distribution ASYMPTOMATIC: no symptoms Isosporiasis Duodenal contents Enterotest (oocysts) Intestinal biopsies Oocysts – visible in direct wet preparations, flotation procedures or concentration - Transparent and can’t be seen in saline wet prep - Seen better in iodin wet prep - zinc sulfate technique or another concentration procedure following polyvinyl alcohol (PVA) preservation. - Auraminerhodamine permanent stain. - Acid-fast stain No intermediate host Both asexual and sexual reproduction take place Ingestion of ineffective mature (sporulated) oocytes (gametogony) takes place in the same intestinal area. Asexual reproduction (schizogony) Immature oocysts typically complete their development in the outside environment. mature sporulated oocyst that is capable of initiating another infection. Isosporiasis An increase in - mild gastrointestinal reported cases began discomfort to severe to occur during and dysentery. following World War II. - weight loss, chronic Specifically, cases diarrhea, abdominal were reported in pain, anorexia, Africa, Southeast weakness, and Asia, and Central malaise America. - Charcot-Leyden crystals, Increase in patients malabsorption suffering from AIDS syndrome - foul-smelling stools Oral anal sexual that are pale yellow contact mode of and of a loose transmission consistency. - Increased fecal fat levels - Infected patients may shed oocysts in their stools for as long as 120 days. Death may result from such severe infections. TREATMENT: - Bland diet and rest - Chemotherapy (w/ trimethoprim and sulfamethoxazole or pyrimethamine and sulfadiazine) proper personal hygiene adequate sanitation practices avoidance of unprotected sex, particularly among homosexual men. PARASITE MORPHOLOGY LAB DIAGNOSIS LIFE CYCLE NOTES EPIDEMIOLOGY CLINICAL SYMPTOMS / TREATMENT PREVENTION AND CONTROL Sarcocystis species Stool specimen Routine histologic methods In addition to its presence in cattle and pigs, Sarcocystis spp. may also be found in a variety of wild animals. adequate cooking of beef and pork Sarcocystis infection. Asexual reproduction of Sarcocystis occurs in the intermediate host. transmission occurs when uncooked pig or cattle meat infected with Sarcocystis sarcocysts is ingested. (Human is definitive host) Transmission route occurs when humans accidentally swallow oocysts from stool sources of animals other than cattle or pigs(human is the intermediate host) *In many cases, only single or double sporocysts cemented together may be visible in stool samples. Infections are relatively low SARCOCYSTIS INFECTION: - experienced fever, severe diarrhea, weight loss, and abdominal pain. It is presumed that patients suffering from muscle tenderness and other local symptoms Treatment: - Definitive host: trimethoprim plus sulfamethoxazole or pyrimethamine plus sulfadiazine - Intermediate host: no known chemotherapy proper care and disposal of animal stool PARASITE Cryptosporidium parvum Cryptosporidosis MORPHOLOGY LAB DIAGNOSIS LIFE CYCLE NOTES EPIDEMIOLOGY CLINICAL SYMPTOMS / TREATMENT PREVENTION AND CONTROL Stool specimen Ingestion of mature oocyst Worldwide distribution Cryptosporidiosis: - Diarrhea: lasts 2 weeks, 1-4 weeks in some daycare centers. - Fever, nausea, vomiting, weight loss, and abdominal pain may also be present. - Great fluid loss may be fatal - Malabsorption Proper treatment of water supplies, use gloves, and wearing gown Iodine or modified acid-fast stain. Formalin fixed smears stained with Giemsa Enterotest,, enzymelinked immunosorbent assay (ELISA) excystation in the upper gastrointestinal tract, Asexual and sexual multiplication Sporozoites rupture Indirect immunofluorescence Thin cell: autoinfection Modified zinc sulfate flotation or Sheather’s sugar flotation Thick cell: remains intact and is passed out of the body may initiate autoinfection occasionally 20 species known only C. parvum infect humans Water or food contamination Person-to-person AIDS patients risk of contracting this parasite Children in tropical areas, animal handlers, travelers abroad TREATMENT: - Spiramycin (experimental) Proper handwashing and disinfecting Enteric precautions PARASITE Blastocystis hominis Blastocystis hominis infection. MORPHOLOGY LAB DIAGNOSIS LIFE CYCLE NOTES EPIDEMIOLOGY CLINICAL SYMPTOMS / TREATMENT PREVENTION AND CONTROL Stool specimen sporulation or binary fission. Epidemics in subtropical countries participates in sexual and asexual reproduction, and exhibits pseudopod extension and retraction. From Saudi Arabia to British Columbia. BLASTOCYSTIS HOMINIS INFECTION: - diarrhea, vomiting, nausea, and fever, as well as abdominal pain and cramping. Proper treatment of fecal material, thorough hand washing Iodine wet preparations Peripheral cytoplasm appears yellow central vacuole is transparent Permanent stain preparations Peripheral cytoplasm dark stain central vacuole not apparent In water or saline lyses may lead to false-negative results ingestion of fecally contaminated food or water. TREATMENT: - Iodoquinol or metronidazole subsequent proper handling of food and water PARASITE Cyclospora cayetanensis Cyclospora cayetanensis infection MORPHOLOGY LAB DIAGNOSIS LIFE CYCLE NOTES EPIDEMIOLOGY CLINICAL SYMPTOMS / TREATMENT PREVENTION AND CONTROL stool samples (concentrated nontraditionally without the use of formalin fixative) ingestion of an oocyst many countries, including the United States and Canada Cyclospora cayetanensis Infection - a longer duration of diarrhea than cryptosporidiosis. - no known connection between C. cayetanensis infection and immunocompromised patients properly treating water sporulate best at room temperature 5% potassium dichromate → sporocysts become visible Flotation methods Modified acid-fast stain Oocysts auto fluoresce under ultraviolet light microscopy. oocyst contains two sporocysts, each enclosing two sporozoites small intestine → sporozoites → asexual reproduction → macroand micro-gametocyte production Male and female gametocytes unite and form oocysts Infected humans pass immature oocysts in the stool. No animal reservoir exists. children living in unsanitary conditions in Lima, Peru travelers and foreigners residing in Nepal and parts of Asia. Contaminated lettuce and fresh fruit using treated water when handling and processing food PARASITE Toxoplasma gondii Toxoplasmosis congenital toxoplasmosis cerebral toxoplasmosis MORPHOLOGY LAB DIAGNOSIS LIFE CYCLE NOTES EPIDEMIOLOGY CLINICAL SYMPTOMS / TREATMENT PREVENTION AND CONTROL blood samples using serologic test Definitive host: cat found worldwide enclosed bradyzoites: transform into tachyzoites (brain or muscle tissue) populations at risk: individuals suffering from AIDS avoidance of contact with cat feces. asexual and sexual reproduction: gut of cat occur in 15% to 20% of the population in the United States. Asymptomatic: - asymptomatic - cause disease in humans when: 1. a virulent strain of the organism has entered the body 2. the host is in a particularly susceptible state (e.g., those suffering from AIDS) 3. the specific site of the parasite in the human body is such that tissue destruction is likely to occur recommended test for the determination of IgM present in congenital infections: doublesandwich ELISA method IgM and IgG – IFA test Other: IgG – IHA and ELISA sexual cycle results in the production of immature oocysts, shed in stool complete maturation: outside environment; 1-5 days rodents – intermediate hosts sporozoites → mature oocysts → tachyzoites (intestinal epithelium) → migration → brain or muscle → cysts w/ bradyzoites human infection: 1. hand-to-mouth transmission 2. human ingestion of contaminated undercooked meat from cattle, pigs, or sheep 3. transplacental infection 4. contaminated blood is transfused into an uninfected person consumption of undercooked meat and its juices by women and their children in Paris was reported in 93% (the highest recorded rate) and 50% 4000 infants born with transplacentally acquired T. gondii infections wearing protective gloves when cleaning out a cat litter box, disinfecting the litter box with boiling water, and thorough hand washing afterward. avoidance of ingesting contaminated meat. avoidance of tasting raw meat all meat should be thoroughly cooked keeping cats away from potentially infective rodents, feeding cats only dry or cooked canned cat food, and/or not having cats at all PARASITE Pneumocystis jiroveci Pneumocystosis atypical interstitial plasma cell pneumonia. MORPHOLOGY LAB DIAGNOSIS LIFE CYCLE NOTES EPIDEMIOLOGY Histologic procedures – Gomori’s methenamine silver nitrate stain presumed that once inside the host, P. jiroveci takes up residence in the alveolar spaces in lung tissue. Areas of particular note include the United States, Asia, and Europe Giemsa and iron hematoxylin stains may be used serologic techniques monoclonal immunofluorescent sputum, bronchoalveolar lavage, tracheal aspirate, bronchial brushings, and lung tissue Mature cysts rupture, producing actively growing, multiplying, and feeding trophozoites. The trophozoites eventually convert into precysts and cysts. May invade spleen, lung, lymph nodes and bone marrow CLINICAL SYMPTOMS / TREATMENT Pneumocystosis: Atypical Interstitial Plasma Cell Pneumonia: - nonproductive cough, fever, rapid respirations, Organism and cyanosis. transmission: transfer - Interstitial plasma cell of pulmonary droplets pneumonia → cause of through direct persondeath in AIDS patients to-person contact - Kaposi’s sarcoma (AIDS patients) People at risk: - Infected malnourished immunosuppressed infants experience poor patients (those w/ feeding, loss of energy, AIDS), children and a rapid respiration rate, those with and cyanosis. predisposing - infiltrate on chest x-ray conditions - Breathing difficulties (PO2 (arterial oxygen May pass through tension) and a normal placenta and cause to low PCO2 (carbon infection in the fetus dioxide tension)) as well as stillbirth - Prognosis is usually poor TREATMENT: - Trimethoprimsulfamethoxazole (Bactrim) - Pentamidine isethionate and cotrimoxazole (alternative) PREVENTION AND CONTROL personal protection from these droplets Protective gear, such as a mask, worn around known infected persons - Roundworms are unembryonated, elongated, cylindrical in shape. With sensory organs known as chemoreceptors The sexes are separate Female may be a. oviparous producing eggs that hatch outside the body b. viviparous giving birth to tiring young c. larviparous larvae bearing i denoting passage of larvae rather than uggs 3 basic morphologic forms: 1. eggs (female sex cells after fertilization) very in size and shape 2. larvae (juvenile worms) typically long and slender 3. adult worms females are longer than males, complete digestive and reproductive system digestive system: 1. mouth hooks, teeth, plates, and other structures 2. buccal cavity tubular or funnel-shaped 3. esophagus a muscular tube that pumps food posteriorly into the intestine 4. intestine a flattened tube with a wide lumen that follows a straight course from the esophagus to the rectum 5. rectum defecation occurs when the anus is dilated and the pseudocoel pressure forces the feces out - - - - - - NEMATODE CLASSIFICATION Based on the presence or absence of caudal chemoreceptor organ Class Enoplea (class Class Rhabditea (Class Adenophorea; class Secernentia; Class Aphasmidea) Phasmidea) - Without caudal with caudal chemoreceptor organ chemoreceptor organ (phasmids): (phasmids) 1. Trichuris trichiura 1. Ascaris lumbricoides 2. Trichinella spiralis 2. Strongyloides 3. Capillaria philippinensis stercoralis 3. Enterobius vermicularis 4. Angiostrongylus cantonensis 5. Filarial worms 6. Hookworms 7. Dracunculus medinensis Classification according to habitat: Intestinal nematodes Extraintestinal nematodes 1. Enterobius 1. Trichinella spiralis vermicularis 2. Dracunculus 2. Trichuris trichura medinensis 3. Ascaris 3. Filarial worms lumbricoides 4. Necator americanus 5. Ancylostoma duodenale 6. Strongyloides stercoralis 7. Capillaria philippinensis LIFE CYCLE NOTES 1. Direct (homogonic) - Require DH but not IH - Homoxenous 2. Indirect (heterogonic) - Require both DH and one or more IH - Heteroxenous LABORATORY DIAGNOSIS - Recovery of eggs, larvae, and occasional adult worms - Cellophane tape prep (around the anal opening) - Stool samples - Tissue biopsies - Infected skin ulcers - Serologic tests PATHOGENESIS AND CLINICAL SYMPTOMS - FACTORS: 1. Number of worms present 2. Length of time the infection persists 3. Overall health of the host - Infections with nematodes may last up to 12 months or longer - Occurrence of reinfections may increase the infection time up to several years and beyond - (with an exception) all the nematodes may cause intestinal infection symptoms at some point during their invasion of the host a. Abdominal pain b. Diarrhea c. Nausea d. Vomiting e. Fever f. Eosinophilia g. Skin irritation h. Skin blister i. Muscle involvement Enterobius vermicularis Trichuris trichiura Trichuris trichiura egg Ascaris lumbricoides Ascaris lumbricoides, decorticated unfertilized egg Ascaris lumbricoides, adult female. Necator americanus Hookworm egg Strongyloides stercoralis Trichinella spiralis Trichinella spiralis larvae in muscle press. Parasite Enterobius vermicularis Pinworm seat worm Morphology Laboratory diagnosis Cellophane tape prep collected from the perianal region Multiple samples may be required to confirm the presence of a light infection and to detect free from infection May be recovered from stool samples Life cycle notes Epidemiology Humas only known host Temperate areas Self-limiting initiated following the ingestion of the infective eggs Digestive tract small intestine (hatch & release young larvae) grow and mature adult worms (in colon) 15k eggs deposit (perianal region) Most common helminth known to cause infection in the US Hand-to-mouth contamination Most contract 4-6 hrs incubation developing eggs achieve infective status RETROINFECTION infective pinworm eggs that migrate back into the host body AUTOREINFECTION infected individuals reinfect themselves bpnlation mating of - Gravid - worms pregnant female worm to child - Morphologic forms in select likely recovered cases ↳ eggs & adult females Clinical Prevention and ETEROBIASIS: pinworm infection - Intense itching - Pruritus ani - Inflammation of the anal and/or vaginal areas - Intestinal irritation - Mild nausea - Vomiting - Irritability - Difficulty sleeping - Minute ulcers - Mild intestinal inflammation - Abdominal pain Proper hand washing symptoms/treatment ASYMPTOMATIC no clinical symptoms TREATMENT - Albendazole - Mebendazole - Pyrantel pamoate control Practice proper personal hygiene Applying an ointment or salve to an infected perianal area to prevent egg dispersal into the environment Avoid scratching the infected area Thorough cleaning of environmental surfaces Total eradication is highly unlikely ( Parasite Morphology Trichuris trichiura Whipworm Laboratory diagnosis Stool (eggs) processed using zinc sulfate flotation method Life cycle notes Epidemiology Ingestion initiates human infection Found in warm climates w/ poor sanitation practices Eggs larvae (small intestine) growth and development otw to intestinal lumen cecum (complete maturation) Macroscopic examination of intestinal mucosa, rectum Resulting adults take up and intestinal residence in the colon, tract ( adult worms) embedding in the mucosa 4-8 years lifespan of adult sulfate flotation worms in untreated infections method zinc Eggs - characterized by presence of hyaline polar plugs Adult male - usually smaller than the adult female. - Contains easily recognizable curled tail 3rd most common helminth Areas in US inc. warm humid south (rural settings) At risk: children and those in psychiatric facilities Clinical Prevention and TRICHURIASIS: whipworm infection: - Heavy infections: 500-5000 worms - In children: ulcerative colitis - 200 worms in children: 1. Chronic dysentery 2. Severe anemia 3. Growth retardation 4. Catch up growth occurs 5. Rectal prolapse 6. Peristalsis - In adults: 1. inflammatory bowel disease. 2. Abdominal tenderness and pain 3. Weight loss 4. Weakness 5. Mucoid or bloody diarrhea Avoidance of defecating directly into the soil symptoms/treatment ASYMPTOMATIC no clinical symptoms TREATMENT - Albendazole - Mebendazole control Practice proper personal hygiene Avoid usage of feces as fertilizer Avoid placing potentially infective hands into the mouth Thorough treatment of infected people Parasite Morphology Ascaris lumbricoides roundworm man Life cycle notes Epidemiology Stool (eggs) Ingestion of infected eggs that contain viable larvae Most common helminth infection in the world In small intestine larvae emerge from the eggs migrate from liver to lungs by entering blood via penetration through the intestinal wall Found in warm climates and areas of poor sanitation ADULT WORMS may be recovered in small intestine, gallbladder, liver, appendix, stool, vomit, external nares large intestinal Roundworm Laboratory diagnosis of Liver lung capillaries alveoli bronchioles pharynx (by cough) swallowed intestine 250k eggs passed in the feces Eggs may survive in 10% formalin fixative used in stool processing Layers of egg: a. Inner - Vitelline membrane b. Middle - Glycogen layer c. Outermost - Albuminous/mammilary coat Decorticated term that describes the lack of - an outer coating mammalian albuminous At risk: children who place their contaminated hands into their mouths, those who ingest vegetables that were grown using contaminated human feces as fertilizer Clinical Prevention and ASCARIASIS: roundworm infection - Single worm: 1. Tissue damage 2. Secondary bacterial infection - Many worms: 1. Vague abdominal pain 2. Vomiting 3. Fever 4. Distention 5. Obstruction of intestine, appendix, liver, bile duct 6. Discomfort in areas where the worms may exit (anus, mouth or nose) 7. Protein malnutrition 8. Pulmonary symptoms 9. Low-grade fever 10. Cough 11. Eosinophilia 12. Pneumonia 13. Asthmatic reactions Proper sanitation symptoms/treatment ASYMPTOMATIC no clinical symptoms TREATMENT - Albendazole - Mebendazole - Other that are designed to get rid the body of the parasitic worms control Practice proper personal hygiene Avoidance of using human feces as fertilizer Parasite Morphology Necator americanus Laboratory diagnosis Stool (eggs) Larvae may matura Man and hatch from the eggs in stool that has been allowed to sit at room temp (without fixative added) New World hookworm Buccal capsule is necessary to determine the specific hookworm organism Anglo stoma - adult hookworm that is characterized by a buccal cavity that contains tooth skin - Buccal capsule is provided with semilunar cutting plates Equipped with an amphidial gland Tail contains a copulatory bursa and copulatory spicule common name: old world hookworm Diseases: Ancylostomiasis and anemia Characteristic shape of adult is letter C Adult worm has 2 pairs of ventral teeth Has a pair of copulatory spicule which is bristle like penetration Life cycle notes Epidemiology 3rd-stage filiform larvae penetrate through the skin e.g. unprotected feet population is infected with hookworms Migrate to lymphatics and blood system Blood carries the larvae to the lungs penetrate the capillaries enters alveoli migrate to bronchioles coughed up (pharynx) swallowed deposited into the intestine Maturation occurs in the intestine Adult females lay 10k 20k eggs/day Found in warm areas Areas w/ poor sanitation practices May be found in SA, Europe, china, Africa, SA, Caribbean Clinical Prevention and TREATMENT: adequate diet rich in iron, protein and other vitamins Proper sanitation symptoms/treatment ASYMPTOMATIC no clinical symptoms. HOOKWORM DISEASE: ancylostomiasis, necatoriasis - Itching at the site (ground itch) - Sore throat - Blood sputum - Wheezing - Headache - Mild pneumonia with cough <500 eggs - Vague mild GI symptoms, slight anemia, weight loss, weakness >5000 eggs - Diarrhea, anorexia, edema, pain, enteritis and epigastric discomfort - Microcytic hypochromic iron deficiency, weakness and hypoproteinemia and mortality TREATMENT - Pyrantel pamoate - Mebendazole - Iron replacement and other dietary therapy control Practice proper personal hygiene Prompt and thorough treatment of infected people Personal protection of people e.g. covering bare feet Parasite Morphology Laboratory Life cycle notes Epidemiology Clinical symptoms/treatment diagnosis Strongyloides sterocoralis Stool (diagnostic eggs) concentrated with zinc sulfate Threadworm Diagnostic rhabditiform larvae may be recovered in fresh stool samples and duodenal aspirates Enterotest Sputum samples control 3 routes Tropical and subtropical regions : 1) Direct a) Indirect 3) Anto infection Areas of the South Appalachian Mountain region DIRECT Rhabditi form larvae - in the passed feces develop directly into ↳ 3rd stage infective the Hari form warm larvae in moist soil , . Serologic tests (inc. ELISA) INDIRECT rhabditiform thread worm larvae passed - into the outside environment & mature into ( adults ↳ non produce - free-living parasitic) eggs that develop into rhabditform larvae ↳ mature → filaform stage Common name: Threadworm - Adult contains short buccal cavity and a long esophagus - Body of the worm is transparent with fine striated cuticle - Ova has a characteristic Chinese lantern shape - Diseases: Strongyloidiasis and Cochin china diarrhea Prevention and AUTO INFECTION Rhabditi form larvae initiates new of → infective larvae t enters the a cycle infection c- lymphatic system Areas of poor sanitation At risk: those who come into skin contact with contaminated soil and those in poor sanitation practices (e.g. psychiatric facilities) ASYMPTOMATIC no clinical symptoms. STRONGYLOIDIASIS: threadworm infection - Diarrhea - Abdominal pain - Urticaria w/ eosinophilia - Vomiting - Constipation - Weight loss - Variable anemia - Malabsorption syndrome - Site of larvae penetration becomes itchy and red - Pulmonary symptoms - Recurring allergic reactions - Immunocompromised people may suffer from severe autoinfections (leads to spread of larvae throughout the body, increase secondary bacterial infections and death) TREATMENT - Ivermectin - Albendazole Practice proper personal hygiene Proper sanitation Proper handling and disposal of fecal material Adequate protection of the skin Treatment of infected people Parasite Morphology Laboratory diagnosis Life cycle notes Epidemiology Clinical symptoms /treatment Prevention and control Trichinella spiralis Trichina Worm Affected skeletal muscle accidental human infection with a Serologic methods parasite whose normal host is an Other indicators: animal (zoonosis) leukocytosis and eosinophilia, elevated consumption of serum muscle enzyme undercooked levels contaminated meat (striated muscle) No known test is Ingested larvae completely 100% t accurate intestine (maturation) t mating to gravid to female migrates adult intestinal snbmnwsa t lays larvae her tire t.no ) egg stage t infant blood enters t travel striated muscle to to unapt cells nurse to granuloma forms to calcification completion of doesn't life cycle larvae T . spiralis occur ceases encysts when Found worldwide except the tropics (Members of the meat-eating populations) TRICHINOSIS, TRICHINELLOSIS Light infection: 1. Diarrhea 2. Slight fever - Heavily infected: May be found in pig, 1. Vomiting deer, bear, walrus 2. Nausea and rate 3. Abdominal pain. Diarrhea Resistant to colder 4. Headache regions 5. Fever - When larvae begin their Feeding of migration: contaminated pork 1. Eosinophilia scraps to hogs 2. Pleural area pain accounts for a 3. Fever major mode of its 4. Blurred vision transmission 5. Edema 6. Cough 7. Death - When larvae is settled in the striated muscle and encystation begins: 1. Muscular discomfort 2. Edema 3. Local inflammation 4. Overall fatigue 5. weakness TREATMENT: - non-life threatening: no meds (get rest, fluids, fever reducers, pain relievers) - life threatening: prednisone, thiabendazole Thorough cooking of meats Proper storage of meats 15° C [59° F] for 20 days or days Avoidance of feeding pork scraps to hogs Parasite Morphology Laboratory diagnosis Life cycle notes Epidemiology Clinical symptoms Prevention and control Dracunulus medinensis Observing infected ulcers Guinea Induced rupture of the infected ulcers by immersing in cool water reveals the firststage larvae worm Ingestion of drinking water contaminated with infected copepods (freshwater fleas) infected ulcer results at the site of the larvae deposit. ulcer ruptures and releases the larvae into the water. Copepods living in the water consume the first-stage larvae, serving as its intermediate host. larvae of Ingestion t intestine - larvae adult → worms to intestinal penetrate Fresh water (step wells) Ponds, human made water holes and standing water Reservoir hosts: inc. dogs GUINEA WORM INFECTION: Dracunculosis, Dracunculiasis - allergic reactions - secondary bacterial infection - disabilities - mortality - painful ulcer (Once the gravid female settles into the subcutaneous tissues and lays her larvae) - subsequent toxic reactions in the ulcer - nodule formation - death and calcification of an adult worm TREATMENT - total worm removal use of properly treated water for consumption boiling water suspected of contamination prohibiting the practice of drinking and bathing in the same water ceasing the practice of allowing standing water to be ingested use a filter (finely meshed filter) educate the people wall to connective tissues or body cavities & gravid female migrate to skin to lay live first stage - larvae to adult the female may body deposit into site at / deeper (becomes escape from the larvae migrate back tissues absorbed ) check steps highly unlikely of total eradication Ancylostoma braziliense - Common name: Cat hookworm - Disease: Cutaneous larva migrans / creeping eruption - Adults have a pair of teeth and a pair of inconspicuous median teeth - Copulatory bursa is broad and long with short lateral rays Ancylostoma caninum - Common name: Dog hookworm - Disease caused: Cutaneous larva migrans/creeping eruption and eosinophilic enteritis - Adult worm has 3 pairs of ventral teeth - Cephalic amphidial gland HISTOLOGY SARCODINA HANDOUT SUBPHYLUM SARCODINA Subphylum Sarcodina Class Lobosea Intestinal Species Entamoeba histolytica Entamoeba hartmanni Entamoeba coli Entamoeba polecki Endolimax nana Iodamoeba b𝑢̈ tschlii Extraintestinal Species Entamoeba gingivalis Naeglaria fowleri Acanthamoeba species • • GENERAL RULES • • • • • SUBPHYLUM: SARCODINA • Organism is generally known as AMEBAE • • • • • • • Entamoeba histolytica – pathogenic (disease causing) Entamoeba dispar – (morphologically the same with histolytica) but different rna and dna Entamoeba moshkovskii – classified as a free-living microorganism. (Morphologically the same with histolytica) o Laredo stain Entamoeba hartmanni - (morphologically the same with histolytica) but can be differentiated through size. o Small race E. histolytica Entamoeba Coli Endolimax nana – smallest amoeba o Comparable to the size of your red blood cells Entamoeba gingivalis – found most likely in the oral cavity Iodamoeba butschii – has a high affinity to iodine Entamoeba polecki – found most likely in pigs and monkeys. • • Commensals – except E. histolytica (pathogenic) Encystation – except E. gingivalis (does not have a cystic stage) Infected Stage: Cyst – except E. gingivalis (does not have a cystic stage) Mode of transmission: ingestion (except for gingivalis: through mouth-to-mouth contact) Habitat: Large intestine (except for gingivalis: oral cavity) Locomotor organelle: Pseudopodium – cell membrane extension Multiplies through: Binary Fission PSEUDOPOD MORPHOLOGIC FORMS: CYST: • • Dominant stage Non-motile • Formed stools TROPHOZOITE • • Feeding stage Vegetative stage BASIC STRUCTURE OF AMEBAE: • • • • • PSEUDOPODS: Often referred to as false feet KARYOSOME: Also referred to as karyosomal chromatin; small central mass of chromatin PERIPHERAL CHROMATIN: A chromatin material that surrounds the karyosome CHROMATOIDAL BARS: Structures that contain condensed RNA material GLYCOGEN MASS: Cytoplasmic area without defined borders that is believed to represent stored food CLASSIFICATION OF INTESTINAL PROTOZOA TRUE AMOEBA • SIGNIFICANCE TWO REASONS: 1. Mistaken for the pathogenic entamoeba histolytica 2. Indication of fecal contamination of food and water ENDOLIMAX NANA • • • • Entamoeba o Peripheral Chromatin o Chromatoidal bodies OTHER AMOEBA • COMMENSAL AMEBAE Endolimax and Iodamoeba o No peripheral chromatin o Chromatoidal bodies are absent DIFFERATIATING AMEBAE • Commensal Smallest amoeba Cross-eyed cyst Cyst o 5 -12 um o Spherical, Ovoid, and Ellipsoid o One – four nuclei o Large and Blot-Like o Cross-eyed cyst Trophozoite o 5 – 12 um o Irregular o Mononucleated o Large and Blot-Like o Granular and vacuolated o Blunt Pseudopods o Sluggish IODAMOEBA BUTSCHLü • • • Commensal Iodine Cyst Large glycogen vacuole • Cyst o 9 – 10um (6-16) o Spherical to Ellipsoidal o Mononucleated Trophozoite o 9-14um (4-20) o Irregular o Mononucleated o Bacteria, yeast cells, and other debris • ENTAMOEBA HISTOLYTICA AMOEBIASIS • • • • • • • Pathogenic Amoebic Dysentery Colitis and Liver abscess Flask-shaped ulceration Gal/ Gal Nac lectin Cysteine proteinases Amebapores VIRULENCE FACTORS • • • • Adhesion molecules (N-acetyl-Dgalactosamine inhibitable lectine Gal/GalNac – adhesion to colonic mucine and host cells o Induce contact dependent cytolysis Channel-forming peptides (Amoebapores): Stored in cytoplasmic granules and release following target cell contact, forms iron exchanging channels in plasma membrane – lysing the target cells Cystein protinases – Aid in penetration of host tissue by digesting extracellular matrix, cleaving collagen, elastin, fibrinogen in extracellular matrix by stimulating host cell proteolytic cascade Resistance to host response o Complement resistanceinactivates the complement factors and are thus resistant to complement mediated lysis. o Limit the effectiveness of humoral response by degrading both IgA and IgG ENTAMOEBA HISTOLYTICA • • • • Cyst o 10-20um o Spherical to round o One- four nuclei o Small and central karyosome o Bull’s eye cyst Fine and evenly distributed Peripheral chromatin Rod. Cigar, sausage, and coffin shaped ASYMPTOMATIC CARRIER STATE • • • • • Trophozoite o 12-60um o Irregular o Mononucleated o Small and central karyosome o Fine and evenly distributed o Peripheral chromatin Hematophagous trophozoite Clean-looking Dendritic or Fingerlike Directional and progressive Entamoeba histolytica THREE FACTOS: 1. Low virulence strain 2. Inoculation into the host is slow 3. Patient’s immune system is intact PHOTOGENESIS AND CLINICAL MANIFESTATIONS: • • • • Amebic colitis Amebic liver abscess Ameboma Flask-shaped ulcer FLASK-SHAPED ULCERATION Bacillary Dysentery May be epidemic Acute onset Prodromal fever and malaise common Vomiting common Patient prostrate Watery, bloody diarrhea Odorless stool Stool microscopy: numerous bacilli, pus cells Macrophages. Red cells, no charcotleyden crystals Abdominal cramps common and severe Tenesmus common Natural history: Spontaneous recovery in a few days, weeks or more; no relapse Amebic Dysentery Seldon epidemic Gradual onset No prodromal features No vomiting Patient usually ambulant Bloody diarrhea Fishy odor stool - FECAL OCCULT BLOOD TEST • Stool microscopy: few bacilli, red cells, trophozoites with ingested red blood cells, charcotleyden crystals Mild abdominal cramps Tenesmus uncommon Natura; history: lasts for weeks; dysentery returns after remission; infection persists for year Occult blood – “Hidden blood” o A. Used for early detection of colorectal cancer o B. Principle – pseudoperoxidase activity of hemoglobin releases oxygen from hydrogen peroxide to oxidize guaiac reagent o Interpretation – blue color indicates gastrointestinal bleeding LABORATORY DIAGNOSIS: • • • • • Direct Fecal smear: o Saline solution: trophozoite motility o Saline sol’n + MB: Entamoeba spp. Will stain blue o Saline sol’n + iodine: nucleus and karyosome can be observed Concentration techniques: FECT & MIFC Culture: Robinson’s and Inoki medium Serologic Testing: ELISA, IHAT, CIE, AGD & IFAT PCR, Ct-scan and MRI CULTURE MEDIA • Witch of whished broomstick • Trophozoite o 15-50um o Irregular shape o Mononucleated o Eccentric karyosome o Irregular peripheral chromatin Dirty-looking o Broader and blunter pseudopodia o Sluggish, non-directional, nonprogressive ENTAMOEBA HISTOLYTICA • • • • • • Robinson’s medium Jones medium Rice egg saline medium Boeck and drbohlav medium Diamond culture medium Balamuth egg yolk infusion Treatment • • Metronidazole Diloxanide furoate Candidate Vaccine Molecules: • • • SREHP Gal/GalNac lectin Cysteine-rich amoebic antigen ENTAMOEBA COLI • • Commensal Harmless inhabitant of the colon • Cyst o 10-35 um o Rounded or elongated o 5-8 nuclei with eccentric karyosome o Irregular peripheral chromatin Splinter needles • • TROPHOZOITE Movement Shape of pseudopodia ENTAMOEBA HISTOLYTICA Unidirectional ENTAMOEBA COLI Blunted Manner or release of pseudopodia Nucleus Cytoplasm Inclusion Size CYST No. of nuclei Chromatoidal bar Nuclear membrane Explosive Uninucleated Dirty looking RBC Smaller ENTAMOEBA HISTOLYTICA Quadrinucleated ENTAMOEBA COLI Broomstick, splinter-like Thin ENTAMOEBA DISPAR • Entamoeba histolytica • Different DNA and Ribosomal RNA • Isoenzyme pattern ENTAMOEBA MOSHKOVSKII • Entamoeba histolytica; Entamoeba dispar • Biochemically and genetically • First detected in sewage • Osmotolerant • Ribodeme 2 ENTAMOEBA HARTMANNI • Entamoeba histolytica • Small race • Cyst o 4-10um o Quadrinucleated o Rod-shaped chromatoid material with rounded or square ends • Trophozoite o 3-10um o Does not ingest red blood cells ENTAMOEBA POLECKI • Rarely it can infect humans • Pigs and monkeys • Cyst o Consistently uninucleated o Angular or pointed chromatoidal bars • Trophozoite o Small karyosome centrally located o Sluggish ENTAMOEBA GINGIVALIS • Found in the mouth • : Kissing, droplet spray, or by sharing of the utensils • Does not undergoes encystation • Trophozoite o Surface of gum, teeth, in gum pockets, and sometimes in tonsillar crypts • 10-20um • Blunt pseudopods • Bacteria o Numerous • Food vacuoles o Mostly leukocytes ENTAMOEBA CHATTONI • Apes and monkeys • Identical entamoeba polecki • Isoenzyme analysis FREE-LIVING PATHOGENIC AMEBAE NAEGOLERIA FOWLERI • Free-living amebae • Primary amoebic meningoencephalitis • Virulence factors: o Phospholipase o Sphingomyelinase • Life cycle stages: o Trophozoite ▪ Ameba o Swimming form ▪ Flagellate o Cyst ACANTHAMEOBA SPP. • Free-living amebae • Acanthamoeba keratitis • Granulomatous amebic encephalitis • Cutaneous acanthamebiasis • Trophozoite stage o Acanthopodia o Thorn-like appendages • Life Cycle stages o Trophozoite o Cyst stage (wrinkled cyst)