Carbon Compounds & Carbohydrates Worksheet
advertisement

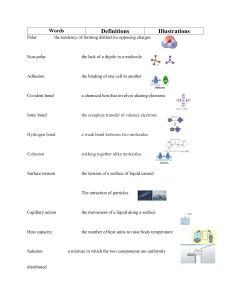
Carbon compounds Carbohydrate Name : ______________________________ Class : ____________ Date: __________ 1. Complete If carbon has ----------------valence electrons, then it can form _______ bond(s). If hydrogen has_____ valence electrons, then it can form ________ bond(s). If oxygen has_____ valence electrons, then it can form _________ bond(s). If nitrogen has_____ valence electrons, then it can form ________ bond(s). 2. What is unique to carbon that makes it the most important element in organic molecules? ___________________________________________________________________ ___________________________________________________________________ 3. What are the four common characteristics of all organic molecules? _________________________ ___________________________ _________________________ ___________________________ 4. How are the building blocks of organic molecules like bricks? __________________________________________________________________ 5. What are the definitions for a monomer and polymer? Monomer: _________________________________________________________ Polymer: _________________________________________________________ 6. Complete the chart below Organic molecule Monomer Polymer Function Elements Examples Carbohydrate 7. For each of the following statement write true or false The formula for all carbohydrate is _________ Carbon atoms can bond together in a straight chain, branched chain or rings. ___________ Large molecules containing carbon atoms are called micromolecules ___________ Milk sugar is the common name for maltose ___________ Sucrose cellulose lactose glucose starch fructose glycogen maltose galactose Monosaccharides Disaccharides 8. Direction: Write each name or formula under the correct heading. Use these items Polysaccharides 1 2 3 Short answers 9. How many rings are in a Monosaccharide? ____________ Disaccarides ? ____________ Polysaccharides ?_______________ 10. What is the most common monosaccharide? _____________________ 11. What is the storage polysaccharide in plants? ________________ In animals? _______________ 12. What is the relationship between glucose, fructose, and galactose? ____________________________________________________________ 13. Why would an athlete have a big pasta dinner the night before a race? ___________________________________________________________ 14. What cellulose used for? _______________________ 15. Label the following diagrams using the terms dehydration – hydrolysis H2O _________________ _____________________