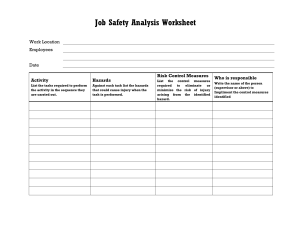
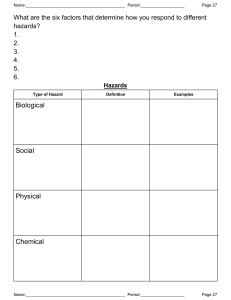
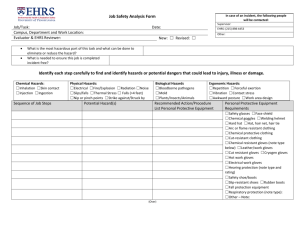
MAINTAINING LABORATORY SAFETY AND SURROUNDINGS MAINTAINING LABORATORY SAFETY By F.S. Mwalilo 2022 CHAPTER ONE Laboratory Safety Responsibilities Responsibilities. Principal Investigators, laboratory personnel, your department and EHS (Environmental Health & Safety) all share responsibility for laboratory safety. Specific duties of each include the following: Department Chair/ Laboratory Manager 1. Ensure the department’s compliance with health and safety standards. 2. Provide timely notification upon termination of facility, who used hazardous materials, to speed up clearance of the laboratory for the next investigator/ manager. 3. Consider appointment of a safety committee from a cross-section of the department’s employees to address departmental safety concerns. Principal Investigator/ Laboratory technician 1. Prepare a Laboratory Safety Plan (LSP), to complement the Laboratory Safety Manual (LSM). These documents constitute your Chemical Hygiene Plan as required by OSHA (Occupational Safety and Health Authority). 2. Ensure that laboratory personnel meet the training requirements of the Laboratory Standard, including chemical hazard information, safety rules and good work practices. 3. Provide initial training to laboratory personnel, upon employment, on the contents of the Chemical Hygiene Plan. Employees document this training through the online Lab Safety Plan application. 4. Provide annual training to all laboratory employees on the contents of the Chemical Hygiene Plan. Employees document this training through the online Lab Safety Plan application. 5. Ensure that staff and visitors observe safety rules and don proper Personal Protective Equipment (PPE) when working in or visiting the laboratory. 6. Ensure that proper safety supplies and equipment, such as gloves, safety glasses and/or goggles, lab coats, and respirators* are available for all people in the laboratory. *Note: if respirators are required. 7. Obtain safety data sheets (formerly known as material safety data sheets) for hazardous chemicals used in the laboratory and make these available to the laboratory staff. 8. Post appropriate hazard information signs within the laboratory. 9. Provide information to EHS in a timely manner so that it may post appropriate signage at laboratory entrances. 10. Conduct an “exit interview” with laboratory workers prior to their departure to ensure that they have properly labelled and prepared hazardous materials for disposal by EHS or use by other workers. 11. Notify EHS prior to vacating laboratory space when moving on campus and notify department chair and EHS of planned departure from UNC or discontinuance of the use of hazardous materials. Decontaminate laboratory surfaces and prepare hazardous materials for disposal by EHS or use by other laboratories. Refer to EHS guidelines on vacating laboratory space. Laboratory Workers /Assistants 1. Study the Laboratory Safety Plan, the chapters of the Laboratory Safety Manual relevant to your research, and other information provided by your supervisor. 2. Complete appropriate training and orientation programs provided by EHS. 3. Complete and submit a Laboratory/Radiation Worker Registration Form to EHS whenever there is a change in work location or laboratory assignment. See Appendix 1-A for instructions on how to fill out this form. 4. Follow safety guidelines when handling hazardous materials, including the proper use of personal protective equipment. 5. Notify EHS of accidents, spills, or conditions that may warrant further investigation and/or monitoring. 6. Review laboratory materials to ensure that you have properly labelled and prepared all hazardous materials for disposal by EHS or use by other workers before you leave the research group. Environment, Health & Safety 1. Provide training and orientation programs for laboratory personnel. 2. Inspect laboratories regularly for safety and health hazards and for compliance with state and federal regulations. 3. Investigate potential safety and health hazards identified by laboratory employees. 4. Monitor personnel for over-exposures to chemical, biological, physical, and radioactive hazards. 5. Advise laboratory personnel on proper disposal of waste chemicals and other hazardous materials. 6. Consult with faculty, staff, students, and departmental safety committees on safety matters. 7. Assist safety committees in organizing committee meetings. 8. Post appropriate signage at laboratory entrances. CHAPTER TWO General laboratory Guidelines and rules: The following recommendations are simply guidelines for safe laboratory practices, and they should not be understood as a complete code of practice. Consult your institution’s safety committee and follow local rules and regulations pertaining to laboratory safety. The below guidelines/ rules are categorised as personal guideline and environmental guidelines 1. Personal safety and Guidelines i. Always wear appropriate personal protective equipment. Change gloves when contaminated, and dispose of used gloves with other contaminated laboratory waste. ii. Wash your hands after working with potentially hazardous materials and before leaving the laboratory. iii. Do not eat, drink, smoke, handle contact lenses, apply cosmetics, or store food for human consumption in the laboratory. iv. Follow the institutional policies regarding safe handling of sharps (i.e., needles, scalpels, pipettes, and broken glassware). v. Take care to minimize the creation of aerosols and/or splashes. vi. Decontaminate all work surfaces before and after your experiments, and immediately after any spill or splash of potentially infectious material with an appropriate disinfectant. Clean laboratory equipment routinely, even if it is not contaminated. vii. Report any incidents that may result in exposure to infectious materials to appropriate personnel (e.g., laboratory supervisor, safety officer). viii. Decontaminate all potentially infectious materials before disposal. ix. Avoid skin and eye contact with all chemicals. x. Assume that all chemicals of unknown toxicity are highly toxic. xi. Post warning signs when unusual hazards, hazardous materials, hazardous equipment, or other special conditions are present. xii. Avoid adding solids to hot liquids. xiii. All laboratory personnel should place emphasis on safety and chemical hygiene at all times. 2. Environmental safety Guidelines i. Do not pour chemicals down drains. Do NOT utilize the sewer for chemical waste disposal. ii. Keep all sink traps (including cup sink traps and floor drains) filled with water by running water down the drain at least monthly. iii. Do not utilize fume hoods for evaporations and disposal of volatile solvents. iv. Perform work with hazardous chemicals in a properly working fume hood to reduce potential exposures. v. Avoid working alone in a building. Do not work alone in a laboratory if the procedures being conducted are hazardous. vi. Laboratory employees should have access to a chemical inventory list, applicable SDSs, Department Laboratory Safety Manual, and relevant SOPs. vii. Access to laboratories and support areas such as stockrooms, specialized laboratories, etc. should be limited to approved personnel only. viii. All equipment should be regularly inspected for wear or deterioration. ix. Equipment should be maintained according to the manufacturer’s requirements and records of certification, maintenance, or repairs should be maintained for the life of the equipment. x. Designated and well-marked waste storage locations are necessary. xi. No cell phone or ear phone usage in the active portion of the laboratories, or during experimental operations. xii. Clothing made of synthetic fibres should not be worn while working with flammable liquids or when a fire hazard is present as these materials tend to melt and stick to exposed skin. xiii. Laboratory coats should not be stored in offices or break rooms as these spreads contaminates to other areas. xiv. Computers and instrumentation should be labelled to indicate whether gloves should be worn or not. Inconsistent glove use around keyboards/keypads is a source of potential contamination. xv. Avoid wearing jewellery in the lab as this can pose multiple safety hazards. CHAPTER THREE Protective Clothing and Equipment: Commonly referred to as PPE (Personal protective equipment), is equipment worn to minimize exposure to hazards that cause serious workplace injuries and illnesses. These injuries and illnesses may result from contact with chemical, radiological, physical, electrical, mechanical, or other workplace hazards. Personal protective equipment may include items such as gloves, safety glasses and shoes, earplugs or muffs, hard hats, respirators, or coveralls, vests and full body suits. 1. Eye and Face Protection Safety Glasses Goggles 2. Use of Gloves Wear proper protective gloves for potential contact with corrosive or toxic materials, materials of unknown toxicity, sharp edged objects, and very hot or cold materials. Select gloves based on the material handled, the particular hazard involved, and their suitability for the operation conducted. While it is important to wear gloves while performing laboratory manipulation of potentially hazardous materials, it is equally important to remove gloves before contacting “clean” areas such as food area surfaces, or common equipment such as telephones, computer keyboards, and photocopiers. Do not wear gloves outside the laboratory, as you could possibly contaminate surfaces you touch such as doorknobs, elevator buttons, or restroom fixtures (Figure 5.3). Remove your gloves even if you believe they are non-contaminated, as others do not know if you might have handled hazardous materials with your gloved hand(s). 3. Laboratory Clothing and Protective Gear The clothing you wear in the laboratory can affect your safety. Do not wear loose (e.g., gown, hanging neckties, oversized or ragged laboratory coats), skimpy (e.g., shorts, halter-tops), or torn clothing in the laboratory. Loose or torn clothing and unrestrained long hair can easily catch fire, dip into chemicals, or become ensnared in apparatus and moving machinery. Skimpy clothing offers little protection to the skin in the event of chemical splash. Finger rings can react with chemicals, and you should avoid wearing them around equipment with moving parts. Appropriate protective gear is advisable for most laboratory work and may be required for some. Such gear can include laboratory coats and aprons, Overall, special boots, shoe covers, and gauntlets, which can be washable or disposable in nature. Commercial garments are available to protect from chemical splashes or spills, heat/cold, moisture, and radiation. Laboratory coats help prevent contact with dirt and the minor chemical splashes or spills encountered in laboratory-scale work. The cloth laboratory coat is, however, primarily a protection for clothing and may itself present a hazard (e.g., combustibility) to the wearer. 4. Foot Protection Wear shoes at all times in laboratories or other chemical use and storage areas. Do not wear holed shoes, sandals, or cloth sneakers in laboratories or mechanical work areas. Safety shoes protect the feet against injuries from heavy falling objects, crushing by rolling objects, or lacerations from sharp edges. 5. Respiratory Protection (Face masks) Respiratory protection might be necessary when working with highly toxic chemicals, biological hazards, or dusts known to cause asthma or pulmonary fibrosis. However, respirators are a “last line” of defence, and should not be used until all engineering controls (e.g. ventilation) and work practice controls (e.g. product substitution) are exhausted. The respirator regulations do not cover “comfort masks” or surgical masks (Figure above). These are technically not respirators, as they are not certified by NIOSH (National Institute for Occupational Safety and Health), and have no protection factor rating. If you are using these masks in the lab, consider whether you might need a true respirator such as those depicted in Figure below. CHAPTER FOUR Proper Storage of Equipment and Chemicals in Laboratories Introduction This chapter instructs you how to interpret the labels on chemical containers, and how to safely store chemicals in the laboratory in a way that minimizes incompatible chemical reactions, spillage, breaking, or waste due to expiration. Inventory and Inspection Label a storage place for each chemical, and return it to that place after each use. Store chemicals by hazard class, not the alphabet, and post storage areas to show the exact location of the chemical groups. Inspect chemical storage areas at least annually for outdated or unneeded items, illegible labels, leaking containers, etc. Examples of chemicals in poor condition, that you should NOT keep stored in your lab: ✓ Expired/outdated chemicals ✓ Illegible/removed labels ✓ Degraded containers ✓ Leaking lids Proper Sealing of Chemical Containers To prevent leakage, odours, or reaction with air, tightly seal all containers of highly toxic, highly volatile, smelly, carcinogenic or reactive chemicals. Make sure that caps and other closures are tight on all hazardous chemicals. A limited exception is freshly-generated mixtures such as acids and organics that may generate gas pressure sufficient to burst a tightly sealed bottle. Use commercially available vent caps or keep the lids loose until sufficient time passes to complete the reactions, and then tightly close the lids. Until all reactions are completed, the contents of the bottle are not waste, but are instead the last step of the chemical procedure. Storage Symbols Most chemical manufacturers include chemical storage symbols on their labels. Many manufacturers use symbols that include a hazard ranking system. Chemical signs, Hazard symbols or warning symbols are recognisable symbols designed to warn about hazardous or dangerous materials, locations, or objects, including electric currents, poisons, and radioactivity. The use of hazard symbols is often regulated by law and directed by standards organizations. Chemical Sign Meaning General Warning The symbol denotes a General Warning and acts as a broad reminder that the area you’re working in is likely to contain hazards and risks and you should work accordingly. Biohazard The equipment or containers that you’re dealing with have likely been in contact with biohazardous materials and therefore run the risk of being contaminated. If a lab is working with infectious agents, this sign should mark the area accordingly. Flammable Material Have the tendency to ignite and should be store accordingly. Keep the chemicals away from flames, sparks, and oxidizing substances. Explosive material It pertains to chemicals in the lab that has explosive properties. Toxic It is a generic sign for toxic/poisonous substances and can cause severe damage when inhaled, absorbed, or swallowed. Oxidizing agents They transfer oxygen to another chemical substrate, which means they can give oxygen to flammable substances. They should be store separately from flammable substances. When dealing with oxidizing agents, you should wear proper lab clothing including gloves and eye protection. Corrosive agents Strong chemicals that corrode into your skin and other substances. A drop of the corrosive agent can cause serious damage. So, wear protective gear at all times when working with corrosive substances. Irritant It refers to substances that can irritate the eyes and toxic when inhaled or swallowed. Protective gear must be worn when dealing with substances tagged as irritants. Health hazard It pertains to substances that can cause serious health damage such as respiratory problems, reproductive toxicity, and carcinogenicity. Cabinets You can use cabinets under hoods and laboratory benches for storage of chemicals. In some cases, laboratory furniture manufacturers design cabinets specifically for storage of flammable and/or corrosive materials. However, do not store laboratory chemicals near or under sinks where there may be exposure to water. Storage of cleaning supplies under sinks is acceptable. Cabinets for chemical carcinogens or highly toxic chemicals should have a lock. Desiccator Jars or Cabinets Desiccator jars and cabinets are useful for storage of air and water reactive, toxic, and malodorous chemicals. In case of especially smelly compounds such as mercaptans, replace the desiccator material with a vapor adsorber (e.g. charcoal) to control odours. Bench Tops and Shelves Chemical storage on bench tops is undesirable, and is vulnerable to accidental breakage by laboratory, housekeeping, and emergency response personnel. Do not store liquids on shelves that are above eye-level. When storing chemicals on open shelves, consider several factors such as compatibility grouping (see below), the container material (plastic or metal versus breakable glass), physical state of the chemical (it’s riskier to store liquids on open shelves compared to solids), the relative toxicity of the chemical, and the height and depth of the shelving. Storage by Compatibility Group Store chemicals in the laboratory according to their compatibility groups. Do not store chemicals in alphabetical order, as this might place incompatible chemicals next to each other (examples include acetic acid and acetaldehyde, sodium cyanide and sulfuric acid, sodium borohydride and sodium chlorate), increasing the potential for accidental mixing of incompatible chemicals. Groups and Chemical Classes Compatibility Group Name Group Chemical Class Group A Inorganic Acids, inorganic acids (except nitric), sulphur, arsenic, Inorganic Salts halides, sulphates, sulphites, thiosulfates, halogens, phosphorus, phosphates Group B Inorganic Bases hydroxides, oxides, silicates, carbonates Group C Organics alcohols, glycols, amines, amides, hydrocarbons, esters, aldehydes, phenol cresols, organic sulphides, organic acids Group D Flammables, Combustibles ethers, aliphatic solvents, aromatic solvents Group E Inorganic Oxidizers borates, chromates, manganates, permanganates, chlorates, perchlorates, chlorites, hypochlorite, hydrogen peroxides, amides, nitrates, nitrites, azides Group F Organic peroxides, azides, hydroperoxides Peroxides and Explosives Group G Reactive air and water reactive, metals and hydrides Group H Cyanides, Sulphides cyanides, cyanates, sulphides, carbides, nitrides Group I Highly Toxics, highly toxic compounds, carcinogens, mutagens, Carcinogens, teratogens Reproductive Toxins CHAPTER FIVE Laboratory Waste Management The characterization, management storage and disposal of laboratory wastes (i.e., chemical waste including hazardous and non-hazardous solid waste, radioactive or mixed waste, biohazardous and medical waste, and universal waste) is regulated and requires strict compliance with regulatory obligations. The Principal Investigator (PI) or lab personnel for each laboratory has overall responsibility for managing the process of: ✓ ✓ ✓ ✓ Characterizing laboratory waste Containerizing laboratory waste Marking/labelling laboratory waste Managing laboratory waste in their laboratory spaces Preceding to waste disposal confirmation and waste pickup by EHS. The Environmental Affairs Section of the EHS Department is responsible for managing the review and characterization of laboratory waste, making the waste determination, as well as the approval of laboratory waste disposal requests from the laboratory PI or lab personnel. EHS personnel conduct the transfer of chemical wastes, radioactive wastes, and mixed wastes from laboratory areas to your organization’s Hazardous Materials Facility for treatment (i.e., bulking) or package consolidation and managed storage prior to the transport to off-site treatment and disposal facilities. After the waste as been characterised and contained the following stage is labelling; When a material has no further use, laboratory personnel must identify the material as an unwanted material by attaching the label shown below. The full chemical name (no formulas, abbreviations or structures) of each component in the waste container must be listed as well as the estimated percentages. The accumulation start date must also be noted when waste is first added to the container. The label must include whether the unwanted material is used or unused. If the size of the container or number of contents does not fit on a label, an associated tracking sheet may be used. Best Management Practices for Chemical Waste In addition to container labelling and accumulation time limit requirements, chemical waste managed within laboratories should follow best management practices for containerization, waste segregation, personnel training, lab cleanouts and emergencies. 1. Containers Waste containers and lids must be in good condition and chemically compatible with the waste inside the container. do not use corks or stoppers. Laboratory beakers, flasks, or plastic milk cartons are not acceptable as waste containers. Metal containers are not acceptable unless they are the original container for the chemical waste being managed (no mixtures). Glass or plastic reagent bottles are generally the most convenient to use. All containers of waste must be kept closed at all times, except: i. When the waste is being temporarily collected in a working container, as described above. ii. When waste is being added to, removed from, or consolidated in the container. iii. When venting of the container is necessary for either the proper operation of laboratory equipment or to prevent the dangerous build-up of extreme pressure that may result from a reaction between the wastes being added. Liquid wastes may be accumulated in glass reagent bottles compatible with the waste. If you generate a large volume of liquid waste, consider 5-gallon carboys for solvent accumulation. Filled containers of liquids must have at least ten percent headspace (roughly 1.5 liters) to accommodate thermal expansion during transport and storage. Store glass waste containers in rubber safety carriers, buckets, or similar containers to protect against breakage and spillage. All waste containers holding 4 liters or less of liquid chemical waste, and all glass containers of liquid chemical waste stored on the floor, require secondary containment. Solid wastes may go into a double-lined cardboard box. Liners must be 1.5 mm or greater polypropylene bags. Do not use biohazard or radiation waste bags for solid waste accumulation, regular, labelled trash bags are preferred. Tie and seal each bag individually. Ethidium bromide-containing solid and semi-solid waste (e.g. used gels) is also collected in double bags within cardboard boxes. Collect liquid ethidium bromide waste in carboys or bottles and manage accordingly. Reactive chemicals must be disposed in their original shipping containers, or in containers provided by EHS. 2. Chemical Waste Segregation Segregate containers of acids and bases from one another in individual, compatible containers while accumulating as waste. Do not discharge acids or bases containing heavy metals to the sanitary sewer, i.e., through the laboratory sinks. Do not mix acids and bases containing heavy metals with other acidic or basic wastes and do not include neutralization disposal of aqueous waste into the sanitary sewer as the last step in laboratory procedures. Package oxidizers separately; store and accumulate away from organics including flammable materials. Oxidizers should never be stored or accumulated adjacent or near any organic substances. 3. Laboratory Cleanouts All laboratories are required to inventory their chemicals at least once a year as part of their laboratory safety plan. Chemicals that are unwanted or no longer needed should be removed from the laboratory and disposed of. 4. Chemical Waste Emergencies If a dangerous situation occurs to a chemical waste in your laboratory, do not touch the container. As best as possible, try and determine the contents and potential hazards and call a supervisor or EHS to report 5. Other Laboratory Wastes Medical Waste (sometimes called biohazard waste) is defined as, “any solid waste that is generated in the diagnosis, treatment, or immunization of human beings or animals, in research pertaining thereto, or in the production or testing of biologicals.” Generators should place solid medical waste (other than sharps) in a container that is: ✓ ≤ 15-gallons (57-L), ✓ closable with a lid, ✓ durable, ✓ labelled with the universal biohazard symbol, ✓ leak-proof ✓ a red-coloured container. The bag should be lined with a plastic bag that is: ✓ autoclavable ✓ orange ✓ labelled with universal biohazard symbol Limit the use of orange plastic bags to medical waste that must be autoclaved before disposal or incinerated. All biohazard bags must be orange in colour. 6. Broken Glass and Other Sharp Objects Place uncontaminated and/or decontaminated glassware and non-regulated sharp objects in a plastic bag within a cardboard box identified with a label indicating: “CAUTION, GLASS AND SHARPS, and NON-HAZARDOUS WASTE ONLY “. 7. Empty Chemical Containers and Recycling Empty glass containers can either be used for waste collection if it is compatible with the waste, or defaced and put in with your glass waste box. If the container once held a P-listed, or acutely toxic substance, dispose as waste. CHAPTER SIX Handling Hazards What are hazards? A hazard is a source or a situation with the potential for harm in terms of human injury or ill-health, damage to property, damage to the environment, or a combination of these. Hazards at work may include noisy machinery, a moving forklift, chemicals, electricity, working at heights, a repetitive job, or inappropriate behaviour that adversely affects a worker’s safety and health. What is risk? A risk is the chance of something happening that will have a negative effect. The level of risk reflects: ✓ The likelihood of the unwanted event ✓ The potential consequences of the unwanted event. What are controls? Controls are the measures put in place to decrease the likelihood or consequences from an unwanted event. They can: ✓ Prevent the unwanted event or reduce the loss of control of the hazard (e.g. Reduce or contain energy release) ✓ Reduce the effects (e.g. Provide shield from hazard; event has happened but emergency response and medical treatment reduce the severity and duration of consequences). Most hazards encountered fall into three main categories: i. ii. iii. iv. Mechanical Hazards Chemical Hazards Biological Hazards Physical Hazards i. Mechanical Hazards Mechanical hazard factors include just about anything inorganic that moves or can injure you. These include many tools, machines and (moving) vehicles, but also, for example, black ice and even high steps or stairs, if they are not secured against falling. Mechanical hazards can be caused by controlled moving unprotected parts; which are freely accessible and form, for example, squeezing points, shearing points, cutting and puncturing points, intake and catching points as well as butt joints, ✓ Dangerous surfaces; such as corners, edges, points, cutting, surfaces with high surface roughness, ✓ Mobile work equipment; for example, in connection with remote controls, guidance systems, reverse driving, driving with restricted visibility, on unpaved ground or with a load changing the centre of gravity. ✓ Uncontrolled moving parts; such as overturning, rolling, sliding or falling parts or detached, bursting or flying parts and media splashing out under pressure or ejected media or working material, slippery surfaces and tripping hazards ✓ Crash; to a lower surface or object. ii. Chemical Hazards Chemical hazards are mainly caused by the characteristics of chemical substances that may cause explosions, fires, or corrosions; or emit poisonous gases or mini particles. Often, chemical substances react negatively when exposed to, or mixed with, other materials or chemical substances. For instance, asbestos particles are usually dispersed in the atmosphere when moved. The types of chemical hazards may be Asphyxiants, Corrosive, Irritants, Sensitizers (allergens), Carcinogens, GHS labels for chemical safety Mutagens, Teratogens, Reactive and Flammable. Routes of Chemical Exposure While the use of chemicals in processes, production, and goods have benefited people in many ways, these chemical substances are also the cause of chemical hazards. There are several routes of chemical exposure as described below. ✓ Inhalation – that is breathing in toxic vapours or small chemical particles ✓ Absorption – such as direct exposure to the skin by touching a chemical substance without any protection such as wearing gloves. ✓ Injection – that is when a sharp contaminated object or needle accidentally penetrates a worker’s body (such as hand or foot) ✓ Ingestion – that is when toxins are accidentally swallowed Handling Workplace Chemical Hazards The following are the recommendations from the most effective to the least effective ways to control chemical hazards given by OSHA (Occupational Safety and Health Administration) are as follows: ✓ Elimination/Substitution – where the need for hazardous chemical usage is completely removed or an alternate less or non-hazardous chemical is used. ✓ Engineering Controls – where employers must implement changes that are physical to the workplace that helps to reduce exposure to the chemical hazard on the workers using or handling hazardous chemical substances. ✓ Administrative and Work Practice Controls – changing how a work task is performed or establishing efficient workplace policies, protocols, processes, and control and monitoring mechanisms. ✓ Personal Protective Equipment (PPE) – using PPE such as respirators, gloves, protective fullbody suits, etc., can help in reducing the workers’ direct contact with the hazardous chemical. iii. Biological Hazards Biological hazards, also known as biohazards, refer to biological substances that pose a threat to the health of living organisms, primarily that of humans. This can include medical waste or samples of a microorganism, viruses, or toxins (from a biological source) that can affect human health. Controlling and handling biohazards ✓ Protecting people from infection Infection can result from ingestion, inhalation or skin penetration. In particular, staff or students having little or no microbiological training should not be exposed to situations in which they may not appreciate the potential hazards. Non-laboratory workers such as cleaners and tradesmen should be given special instruction if they are to come into contact with this class of hazard. No one should be working in a microbiological environment without knowledge of recommended practices and procedures. ✓ Preventing cross-contamination of results Prevention of cross-contamination or contamination with adventitious micro-organisms is important since this may nullify experimental procedures and lead to erroneous results. Such a situation may result in incorrect treatment of patients or modified techniques. ✓ Warning signs A biological hazard must be clearly indicated by standard biological warning signs giving the type and degree of risk and the person responsible. Immediately adjacent to the symbol, a sign shall also be displayed stating: Danger - infectious material. ✓ Separate areas Separate areas should be set aside for: i. preparation of media ii. holding of materials iii. sterilisation iv. storage of sterile articles v. collection of specimens from patients vi. receipt of samples – spill trays should be provided. vii. Animal rooms must be segregated from laboratories and should contain separate areas for infected animals, for non-infected animals and post-mortems. ✓ Protective clothing Protective clothing should be worn in microbiological laboratories, and gowns or coats removed before leaving the laboratory for common rooms, office areas or home. ✓ Protective gloves should be worn in highly infective situations, and personnel should disinfect their hands before and after using gloves, as minute holes may allow entry of microorganisms. ✓ Elbow or foot operated taps should be available for washing as well as emergency showers. Wounds and infections provide excellent routes for further infections. Any cut or abrasion should be treated immediately and covered with a waterproof dressing. Any infections, particularly of the respiratory or alimentary tracts or hand wounds, must be reported immediately. ✓ Safety cabinets Clean workstations, using biological safety cabinets or laminar flow clean air benches, are to be used for product and/or user protection. These types of equipment have different purposes and laboratory staff should be aware of the differences. ✓ Biosafety cabinet Virulent pathogenic organisms must be handled in a biosafety cabinet where contaminated air is passed through a high efficiency particulate air (HEPA) filter. There are three types of biological safety cabinet, Class I, Class II and Class III: Class I: inward flow of air away from the operator and a HEPA filter is used before exhaust air is discharged from the cabinet Class II: protects the operator by use of an air barrier and in addition a flow of filtered air passed over the work to prevent it becoming contaminated. A HEPA filter is used before exhaust air is discharged from the cabinet. Class I and II cabinets are completely free-standing and must not be directly connected to ducting which has outside vents as wind may interfere with operator protection. Class III: completely enclosed unit with built-in air locks for introducing and removing materials. Both incoming and outgoing air passes via HEPA filters. This class of safety cabinet is used for work with high-hazard micro-organisms. Any procedure, such as using blenders, shakers or sonicators, that is likely to produce infectious aerosols should be carried out in a biosafety cabinet. All biological cabinets must be inspected and maintained by a registered repairer every 12 months. Back to top ✓ Decontamination Disinfectants; Whenever possible, decontamination should be achieved by sterilisation in an autoclave (steam heat under pressure). Disinfectants should only be utilised where sterilisation is not possible, for example, in large spaces, surfaces and delicate instruments. Disinfectants should be chosen on their effectiveness to deal with the specific type of microorganism. The main uses for disinfectants are: i. ii. iii. washing – such as, discarded containers, re-useable pipettes wiping down benches and work surfaces at end of day regular cleaning of equipment – such as water baths, incubators, centrifuges, freezers, refrigerators. Steam heat autoclaves are used for sterilisation. Only properly trained staff should use the autoclave and care must be taken to ensure the load reaches the required temperature and remains at that temperature for the prescribed time. ✓ Waste disposal All infectious wastes should be disposed of in accordance with both federal and state regulations, and the following procedures should be followed: i. All contaminated waste material shall be sterilised, preferably by autoclaving, before disposal, preferably by incineration. ii. Culture or fluids which may contain viable organisms shall not be poured into sinks or drains. iii. iv. Solid contaminated materials shall not be placed in waste bins. v. Aerosol cans or other sealed containers may explode if autoclaved or incinerated and must be surface sterilised only (using a suitable procedure). vi. Re-useable contaminated glassware should be disinfected or autoclaved or both before cleaning. All samples, remains, disposable equipment, animal carcasses, tissue, fluids, faeces and bedding should be regarded as contaminated. iv. Physical Hazards Physical hazard is an agent, factor or circumstance that can cause harm with contact. They can be classified as type of occupational hazard or environmental hazard. Physical hazards include ergonomic hazards, radiation, heat and cold stress, vibration hazards, and noise hazards. As with chemical hazards, having good awareness of these hazards, good preplanning, use of personal protective equipment and following basic safety rules can go a long way in preventing accidents involving physical hazards. Ergonomic hazards are physical conditions that may pose a risk of injury to the musculoskeletal system. Ergonomic hazards include awkward postures, static postures, high forces, repetitive motion, or short intervals between activities. The risk of injury is often magnified when multiple factors are present. Factors such as whole-body or hand/arm vibration, poor lighting, poorly designed tools, equipment, or workstations all contribute to negative interactions with the worker/user. Some of the common body regions where injuries may occur include, but are not limited to; ✓ Muscles or ligaments of the lower back. ✓ Muscles or ligaments of the neck. ✓ Muscles, tendons, or nerves of the hands/wrists. ✓ Bones and muscles surrounding the knees and legs. Hazard analysis and identification To make your work at your laboratory safe, we start with a risk analysis and identification. It is an essential part of the risk assessment. In cooperation with you and / or your superior, we as safety personnel (if necessary, also the company doctor) look at your workplace and try to determine whether and if so, which hazards can occur. Your supervisor prepares the risk assessment and the safety engineers advise him/her on this. So-called hazard factors can be noise, dust exposure or mechanical factors such as sharp surfaces and edges. The analysis begins with an inventory. This can possibly take place within the framework of a so-called safety inspection or simply an inspection. An inspection is indeed a search for defects, but not with the aim of finding errors or even identifying culprits. Rather, it is a matter of improving working conditions and eliminating possible sources of danger or developing and later implementing measures for improvement in cooperation with the heads of the specialist areas and you as an employee. Inspections are announced in good time and take place together with as many participants as possible. Hazard/ risk evaluation Now it is time to consider whether there is a risk. In order for a hazard to become a concrete hazard, the source of the hazard must coincide with you in terms of space and time. Risk is the combination of the probability of an event occurring and the possible severity of damage or illness. In many cases there are limit values or so-called workplace limit values that can be measured directly. These include, for example, noise exposure, radiation dose, temperature, humidity, etc. In these cases, measurements can be used to determine precisely whether there is a risk or not. But what does it look like if an exposure cannot be measured? Risk assessment As described in the introduction, this table compares the probability of occurrence with the possible severity of a loss. At the points of intersection, a score can then be read, or the colours green, yellow and red can be used to determine whether no measures are necessary (green), whether measures should be considered (yellow) or whether there is even an immediate need for action (red). Let's assume you are travelling by plane: as long as you are not sitting in it, nothing can happen (the source of danger, the plane, must coincide with you in space and time). When you take off, the plane must crash to cause damage. The example is extreme, but illustrates the proportionality. The probability of a crash is extremely low (aircraft are considered the safest means of transport). However, the consequence, i.e. the damage caused by a crash, would be fatal. Now you weigh up and ask yourself: "Do I accept the risk? Have you ever asked yourself this question before a flight? There's no such thing as no risk. Taking no risks is impossible. The risk is either very low, medium or high, or everything in between. The aim of modern occupational safety is to keep the risk as low as possible and, if necessary, to reduce it to an acceptable level. Measurement on the assessed risk/ hazard As soon as a risk has been identified that is no longer acceptable, i.e. outside the "green zone", measures must be taken to reduce the risk. However, measures cannot be taken randomly. They must be well-considered, practicable and, above all, viable. A measure that cannot be implemented because, for example, it is not accepted by employees is ineffective. A measure that would go beyond the company's budget cannot be the goal. When it comes to defining measures, you need to take a measure. The hierarchy of measures As soon as a risk has been identified that is no longer acceptable, i.e. outside the "green zone", measures must be taken to reduce the risk. This follows a so-called hierarchy of measures, which is divided into 5 levels in modern occupational health and safety: Stage 1: Elimination of the source of danger Here the source of danger is to be eliminated completely. This is usually done by replacing the source with a less dangerous one. Can a hazardous chemical be replaced by another non-hazardous one? Stage 2: Elimination of hazards through technical measures Can a source of danger be limited, e.g. by shielding? A fume hood with extraction would be an example in the laboratory. Stage 3: Organisational measures (local and temporal separation from the source of danger) This concerns you personally. If you are exposed to a noise source for only a short time and not permanently, the risk is reduced. Stage 4: Personal protective equipment (PPE) If contact with the source cannot be avoided by any of the above steps, you must wear protective clothing as a measure to separate you or your body from the source of danger. Safety glasses, respiratory protection or hearing protection are typical examples. Stage 5: Behavioural measures This includes, for example, instructions and operating instructions. You must know which substances you handle and which hazards they pose. You will be instructed in how to protect yourself. Important: The effectiveness of the measures decreases steadily from level 1 to level 5. It should always be checked whether a more effective measure is possible. So, it always starts with 1! Only if this is not possible, one goes over to the next stage. When weighing up the measures, you as a human being are always in the centre. It must be weighed whether and how a measure can be realized. Economic aspects also play a role. In addition, the purchase of a new machine or the modification of a workflow must not lead to new, previously unknown or even greater risks! All further steps: They include the implementation of the measures and their control. Do the measures work at all or do they have to be reworked somewhere? You are a key figure here, because you work daily at your previously assessed workplace. CHAPTER SEVEN HANDLING FIRE ACCIDENTS Handling different types of fire Fire is a chemical reaction in which energy in the form of heat is produced. The chemical reaction is known as combustion. Combustion occurs when fuel or other material reacts rapidly with oxygen, giving off light, heat, and flame. A flame is produced during the ignition point in the combustion reaction and is the visible, gaseous part of a fire. Flames consist primarily of carbon dioxide, water vapor, oxygen, and nitrogen depending on the heat given off. The process of existence of fire is summarised in by the fire triangle; that includes the three components that must be present for a fire to burn. These components are: 1. Fuel 2. Oxygen 3. Heat/ ignition source Without one of these components, fire cannot exist. For a fire to ignite. Firefighting equipment/ fire protective equipment is equipment designed to extinguish fires or protect the user from fire. It may be used by trained fire fighters, untrained users at the scene of a fire, or built into a building's infrastructure (such as a sprinkler system). Firefighting equipment includes not only fire hoses and fire extinguishers but also fire-resistant protective clothing, fire-resistant gloves, respirators, fire blankets, helmets etc. Classes of fire and Fire extinguishers Fires can be placed into different classes depending on what material is burning. This classification then gives us information on the type of fire extinguisher we should use to put out the flames. Not all fires require the same type of extinguisher and the correct type has to be used for each blaze. Type of fire Class A Class B Class C Description and extinguisher A class A fire is burning flammable solids as fuel. Examples of these include paper and wood. Extinguishers that can be used: Water, Foam, ABC Dry powder, Wet chemical Class B fires are burning flammable liquids. Examples include petrol and paint. Extinguishers that can be used: Foam, CO2 Gas, ABC Dry powder Class C fires burn flammable gases. A couple of examples are propane and butane. Extinguishers that can be used: ABC Dry powder Class D Electrical (class E) Class F Class D fires are burning flammable metals. These may include lithium or magnesium. Extinguishers that can be used: Dry Special Powder Any fire involving electrical equipment is classed as an electrical fire. Extinguishers that can be used: CO2 Gas, ABC Dry powder Class F fires are burning cooking oils or fat. Extinguishers that can be used: Wet chemical Fire extinguishers are tools/ equipment designed to tackle specific types of fire. There are several different types of fire extinguishers. 1. Water extinguishers: Water extinguishers are one of the most cost-effective ways to fight Class A fires, those fuelled by solid materials such as paper, wood and textiles. There are four different types of water extinguishers: water jet, water spray, water with additives and water mist or fog. ✓ Water jet extinguishers work by spraying a jet of water at the burning materials, cooling them and preventing re-ignition. They should not be used on live electrical equipment. ✓ Water spray extinguishers use a very fine spray of water droplets, each droplet is surrounded by air which is non-conductive. ✓ Water extinguishers with additives are water extinguishers with foaming chemicals added. The water loses its natural surface tension meaning that it can soak into the burning materials more effectively. ✓ Water mist, or fog, extinguishers apply water in the form of mist, or fog, the droplets are much smaller than those from the water spray extinguisher. All water extinguishers have a red label. 2. Foam extinguishers: can be used on Class A and B fires. They are most suited to extinguishing liquid fires such as petrol or diesel and are more versatile than water jet extinguishers because they can also be used on solids such as wood and paper. Foam extinguishers have a cream label. 3. Powder extinguishers: are a good multi-purpose fire extinguisher because they can be used on Class A, B and C fires. They can also be used on fires involving electrical equipment however, they do not cool the fire so it can re-ignite. They are not generally recommended for use inside buildings unless there is absolutely no alternative. Powder extinguishers have a blue label. 4. Carbon dioxide extinguishers (CO2): are ideal for places with a lot of electrical equipment such as offices or server rooms because they are safe to use on fires involving electrical apparatus. They can also be used on Class B fires, those involving flammable liquids such paraffin or petrol. Carbon Dioxide Extinguishers (CO2) have a black label. 5. Wet chemical extinguishers: are suitable for use on Class F fires involving cooking oils and fats, such as lard, olive oil, sunflower oil, maize oil and butter. Although they are primarily designed for use on Class F fires, cooking oils and deep fat fryers. They can also be used on Class A fires (wood, paper and fabrics) and Class B fires (flammable liquids). Wet chemical extinguishers have a yellow label. 6. Fire blankets: are primarily for use on hot oil fires such as frying pans or small deep fat fryers. They can also be used on someone whose clothing has caught fire. They work by smothering the fire, stopping access to the oxygen fuelling it and extinguishing it. Fire extinguisher checking and servicing A fire extinguisher needs to work straight away when needed, so it is vital they are regularly checked and serviced. Fire extinguishers are pressurised vessels that can burst when corroded or damaged and have been known to cause serious injury and even death. The Regulatory Fire Reform (Fire Safety Order) 2005 states that firefighting equipment “must be subject to a suitable system of maintenance and are maintained in an efficient state, in efficient working order and in good repair.” There are two types of maintenance procedures. i. Visual inspection by the user ii. Maintenance by a competent person. Fire extinguishers maintenance intervals The table below shows the recommended intervals for maintenance of fire extinguishers. It may be necessary to carry out a basic service more than once in a 12-month period if the monthly visual inspection identifies any damage or drop in pressure. Extinguisher type Water and water based Powder Powder - primary sealed CO2 Visual inspection Monthly Monthly Monthly Monthly Basic service Annually Annually Annually Annually Extended service 5 years 5 years 10 years 10 years Visual basic inspection The visual inspection should be carried out, and recorded, on a monthly basis and cover: ✓ ✓ ✓ ✓ Location of the extinguisher - is it in the right place? Visibility of the extinguisher - is it positioned in such a way that it can be easily seen? Operating instructions - are the instructions facing outwards, clean and easy to read? The condition of the extinguisher - has it been used, is there any obvious damage or are there any missing parts? ✓ Extinguisher pressure - is the pressure of the extinguisher within safe operating limits? ✓ Tamper seals - have the seals and tamper indicators been broken? Extended servicing Every five years most fire extinguishers need an extended service. This means completely discharging the extinguisher, checking for internal corrosion, refilling and repressurising. Extinguishers should be available for immediate use at all times. Fire extinguishers should be permanently mounted on brackets, floor stands or in extinguisher cabinets in conspicuous positions. Siting the extinguishers in conspicuous positions means they can be easily seen by people following an escape route. This could be near to room exits, corridors, stairways, lobbies and landings. Firefighting rules and regulations 1. Keep flammable liquids to a minimum, and stored in flammable liquid storage cabinets. Never allow more than ten gallons of liquid to be outside of cabinets at any time. 2. Store compressed gases with valve caps on when not in use, and keep cylinders firmly anchored in place. 3. Store incompatible substances in separate areas, keeping oxidizers well away from flammable liquids and gases. 4. Do not store flammable liquids in fume hoods. Importance of checking and servicing fire extinguisher 1. To avoid loss of life and property damage. 2. To ensure that it will properly operate in case of an emergency. 3. To check any signs of damages on the cylinder. Corrosion to the steel extinguishers can occur on the inside, weakening the integrity of the cylinder over time. 4. Possible of hose blockage. Another difficult problem is that the hose might have been clogged, impairing its functionality. The O-rings on the hose can also deteriorate and compromise the extinguishers. 5. Inspection of the pressure gauge. For each working fire extinguishers, there is a pressure meter that indicates its functionality and it has to be centered in the green zone. The meter will drop after a while whether it’s used or unused. 6. Some may need a refill. The extinguishing agents in a fire extinguisher will need a refill after each use, to maintain an optimum spread whenever there’s a fire. Importance of handling fire accidents 1. 2. 3. 4. 5. Reduce the risk of injury to employees and customers Reduce damage to facility/building Protect against possible fines Protect against losing customers’ trust Protect employee jobs that would be lost due to extensive building damage CHAPTER SIX Performing First Aid First aid: refers to the emergency or immediate care you should provide when a person is injured or ill until full medical treatment is available. For minor conditions, first aid care may be enough. For serious problems, first aid care should be continued until more advanced care becomes available. The decision to act appropriately with first aid can mean the difference between life and death. Begin by introducing yourself to the injured or ill person. Explain that you are a first aid provider and are willing to help. The person must give you permission to help them; do not touch them until they agree to be helped. If you encounter a confused person or someone who is critically injured or ill, you can assume that they would want you to help them. This is known as “implied consent”. If you encounter an emergency situation, follow these three basic steps/ procedures for first aid administration: 1. Taking immediate action: This is the key to the ‘Preserving Life’ principle – a quick response to an accident can save lives and minimise the risk that things get worse. If someone needs help, either from an injury or sickness, you shouldn’t hesitate to help if possible. 2. Calming down the situation: First aiders should be able to remain calm under pressure and help reduce the overall stress levels of the injured person as well as other people who may be concerned. Reassurance can provide more support that you might expect in an emergency situation and help people make the right decisions. 3. Calling for medical assistance: Make sure to get a hold of the emergency services as soon as possible, either by calling directly yourself or asking a bystander to do so if you’re preoccupied handing the injury. This will ensure that a medical professional arrives quickly to handle the situation in a more comprehensive manner and provide more specialist treatment. 4. Apply the relevant treatment: Before a medical professional does arrive, you will need to apply first aid treatments in order to stabilise the condition of the injured person. This comes under the ‘preserve life’ banner. 5. Scene safety: Assessing the safety of the surroundings is critical when approaching any scene. You do not want to become another person who is injured or ill, so look for any potential dangers. Remove the person from any dangers, such as the presence of water at the scene. Be especially alert to avoid danger from automobile traffic. Handwashing and personal protective gear Handwashing is essential in the prevention of disease and illness. Wash your hands after each episode of care and after taking off gloves. When a sink is not available, use hand sanitizers. (Most hand sanitizers are alcohol-based and are a substitute for handwashing when needed.) Hand Washing Proper handwashing technique is fairly simple: ✓ ✓ ✓ ✓ Completely wet your hands and generously apply soap. Rub vigorously for at least 20 seconds. Rinse your hands with plenty of running water. Dry your hands with a towel or air dryer. Using personal protective gear is an important strategy to minimize the risk of blood and bodily fluid exposure. If the person is bleeding, always wear gloves and protective eyewear when giving first aid care. This is to reduces the risk for both the rescuer and the injured/ill person to be exposed to a bloodborne disease. Gloves protect your hands from exposure to blood and other bodily fluids, while eye protection prevents accidental exposure from splashing fluids. Consider a pocket mask as part of your personal protective gear as it provides safety during rescue breathing. Be sure to dispose of all equipment that has touched bodily fluids in a biohazard bag when available. When taking off the gloves, avoid touching the outer contaminated surface. Slowly pull one glove off while turning it inside out. Place the glove in the palm of the other gloved hand, and then remove the second glove while turning it inside out. First aid kit First Aid Kit: a small box containing items used in giving help to a sick or injured person until full medical treatment is available. A standard first aid kit should include: ✓ adhesive bandages of assorted sizes ✓ roller bandages of assorted sizes ✓ absorbent compress dressings ✓ sterile gauze pads ✓ adhesive cloth tape ✓ antiseptic wipes ✓ aspirin ✓ acetaminophen or ibuprofen ✓ antibiotic ointment ✓ hydrocortisone cream and calamine lotion ✓ nitrile or vinyl gloves ✓ safety pins ✓ scissors ✓ tweezers ✓ thermometer ✓ breathing barrier (masks) ✓ instant cold pack ✓ blanket ✓ first aid manual First aid bandage In many cases, you can use an adhesive bandage to cover minor cuts, scrapes, or burns. To cover and protect larger wounds, you might need to apply a clean gauze pad or roller bandage. To apply a roller bandage to a wound, follow these steps: ✓ ✓ ✓ ✓ Hold the injured area steady. Gently but firmly wrap the bandage around the injured limb or body part, covering the wound. Clip the bandage with sticky tape or safety pins. The bandage should be wrapped firmly enough to stay put, but not so tightly that it cuts off blood flow. ✓ To check the circulation in a bandaged limb, pinch one of the person’s fingernails or toenails until the colour drains from the nail. If colour doesn’t return within two seconds of letting go, the bandage is too tight and needs to be adjusted. First aid for burns If you suspect that someone has a third-degree burn, call emergency service. Seek professional medical care for any burns that: ✓ cover a large area of skin ✓ are located on the person’s face, groin, buttocks, hands, or feet ✓ have been caused by contact with chemicals or electricity To treat a minor burn, run cool water over the affected area for up to 15 minutes. If that’s not possible, apply a cool compress to the area instead. Avoid applying ice to burned tissue. It can cause more damage. Over-the-counter pain relievers can help relieve pain. Applying lidocaine or an aloe vera gel or cream can also reduce discomfort from minor burns. To help prevent infection, apply an antibiotic ointment and loosely cover the burn with clean gauze. Find out when you should contact a doctor for follow-up care. First aid CPR If you see someone collapse or find someone unconscious, call emergency service. If the area around the unconscious person seems safe, approach them and begin Cardiopulmonary resuscitation (CPR). Even if you don’t have formal training, you can use hands-only CPR to help keep someone alive until professional help arrives. Here’s how to treat an adult with hands-only CPR: ✓ Place both hands on the centre of their chest, with one hand on top of the other. ✓ Press straight down to compress their chest repeatedly, at a rate of about 100 to 120 compressions per minute. ✓ Compressing the chest to the beat of “Staying Alive” by the Bee Gees or any hip hop songs that can help you count at the correct rate. ✓ Continue performing chest compressions until professional help arrives. ✓ Learn how to treat an infant or child with CPR and how to combine chest compressions with rescue breathing. First aid for bee sting For some people, a bee sting is a medical emergency. If a person is having an allergic reaction to a bee sting, call emergency service. If they have an epinephrine auto-injector (like an EpiPen), help them find and use it. Encourage them to remain calm until help arrives. Someone who’s stung by a bee and showing no signs of an allergic reaction can usually be treated without professional help. ✓ If the stinger is still stuck under the skin, gently scrape a credit card or other flat object across their skin to remove it. ✓ Then wash the area with soap and water and apply a cool compress for up to 10 minutes at a time to reduce pain and swelling. ✓ To treat itching or pain from the sting, consider applying calamine lotion or a paste of baking soda and water to the area several times a day. First aid for nosebleed To treat someone with a nosebleed, ask them to: ✓ ✓ ✓ ✓ ✓ Sit down and lean their head forward. Using the thumb and index finger, firmly press or pinch the nostrils closed. Continue to apply this pressure continuously for five minutes. Check and repeat until the bleeding stops. If you have nitrile of vinyl gloves, you can press or pinch their nostril closed for them. If the nosebleed continues for 20 minutes or longer, seek emergency medical care. The person should also receive follow-up care if an injury caused the nosebleed. First aid for heatstroke When your body overheats, it can cause heat exhaustion. If left untreated, heat exhaustion can lead to heatstroke. This is a potentially life-threatening condition and medical emergency. If someone is overheated, encourage them to rest in a cool location. Remove excess layers of clothing and try to cool their body down by doing the following: ✓ Cover them with a cool, damp sheet. ✓ Apply a cool, wet towel to the back of their neck. ✓ Sponge them with cool water. Call emergency service if they develop signs or symptoms of heatstroke, including any of the following: ✓ ✓ ✓ ✓ ✓ nausea or vomiting mental confusion fainting seizures a fever of 104°F (40°C) or greater If they’re not vomiting or unconscious, encourage them to sip cool water or a sports drink. First aid for heart attack If you think someone might be experiencing a heart attack, call emergency service. If they’ve been prescribed nitro-glycerine, help them locate and take this medication. Cover them with a blanket and comfort them until professional help arrives. If they have difficulty breathing, loosen any clothing around their chest and neck. Start CPR if they lose consciousness. It’s also smart to include a list of your healthcare providers, emergency contact numbers, and prescribed medications in your first aid kits. Outlook It’s important to protect yourself from contagious illnesses and other hazards when providing first aid. To help protect yourself: i. ii. iii. iv. v. Always check for hazards that could put your safety at risk before approaching a sick or injured person. Avoid direct contact with blood, vomit, and other bodily fluids. Wear protective equipment, such as nitrile or vinyl gloves when treating someone with an open wound or a breathing barrier when performing rescue breathing. Wash your hands with soap and water immediately after providing first aid care. In many cases, basic first aid can help stop a minor situation from getting worse. In the case of a medical emergency, first aid might even save a life. If someone has a serious injury or illness, they should receive follow-up care from a medical professional. The Principles and Practices of First Aid 1. Preserve Life: The first aim of first aid is to preserve life, which involves the key emergency practices to ensure that the casualty isn’t in any mortal danger. Remember though, this includes preserving your own life as you shouldn’t put yourself in danger in order to apply first aid. Its at this stage where you should do a quick risk assessment to check for dangers to the injured person, yourself or bystanders which could cause the situation to escalate. If in doubt, do not attempt to apply first aid and immediately call for a medical professional. 2. Prevent Deterioration: Once you’ve followed all the steps associated with the first principle, your next priority is to prevent deterioration of the injured person’s condition. Keeping a casualty still to avoid aggravating their injury, or from complicating any unseen issues, is crucial. This helps prevent to further injuries, and clearing the area of any immediate dangers will help you to do so. 3. Promote Recovery: Finally, there are steps you should follow which will help lessen the amount of time taken for a casualty to recover from an accident and aid in minimising lasting damage and scarring. The prime example of this is applying cold water to a burn as soon as possible to lower the chance of long-term scarring and helps speed up the healing process. Check for consciousness 1. 2. 3. 4. 5. Open the airway Check for breathing Follow airway, breathing, of resuscitation, administer CPR if needed Check for circulation Check for bleeding, controlling any major bleeding There are a number treatments which correspond to the different problems that might arise as you work through this list, e.g. CPR, applying a tourniquet, running a burn under cold water, etc. First Aid Regulation/ law Under the Health and Safety at Work etc Act 1974 (HSWA), employers are responsible for making sure that their workplace has a health and safety policy. This should include arrangements for first aid. Employers should also be aware of the Health and Safety (First Aid) Regulations 1981. This places a responsibility on all employers (no matter the size of their business) to provide adequate resources to those who are injured at work. This includes ensuring there is equipment, facilities and first aiders who have had appropriate training. Although the 3Ps are outlined above, we will also include two more areas that needs attention when conducting primary emergency care: 1. 2. 3. 4. 5. Protection against further injury. Preservation of life. Promotion of recovery. Prevention of injuries for people at any age. Promotion of healthy lifestyles.