Kinetic Molecular Model: Liquids & Solids - Chemistry Activity
advertisement

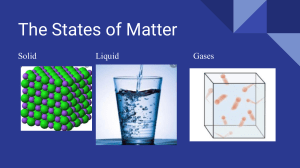
WEEKLY LEARNING ACTIVITY SHEETS General Chemistry 2 & Grade 11, Quarter 3, Week 1(Part 1) The Kinetic Molecular Model of Liquids and Solids Name: Section: Most Essential Learning Competency (MELC) Use the kinetic molecular model to explain the properties of liquids and solids. STEM_GC11IMFIIIa-c-99 Learning Objectives: The learners will be able to: 1. describe the characteristic properties that differentiate liquids and solids; 2. apply the kinetic molecular theory to describe liquids and solids. Time Allotment: 1 hour Key Concepts Phases of Matter Gases, liquids, and solids are made up of representative particles (atoms, molecules, and ions), but those particles' behaviors differ within the three phases. Slideplayer.com Figure 1. Comparison of gases, liquids, and solids at the Molecular level Kinetic Molecular Theory (KMT): The thought that particles of matter are always in motion, which this motion has significant effects. 1. A theory developed within the late 19th century to account for the behavior of the atoms and molecules that structure matter. 2. It supported the thought that particles of matter are always in motion, which has consequences. 3. It is often used to describe the properties of solids, liquids, and gases in terms of particles' energy and the forces that act between them. Three Assumptions of KMT: 1. All matter (solid, liquid, gas) is formed of particles (atoms, ions, molecules). 2. Particles are in continuous motion, which shows the movement of electrons. • Gas particles travel in a completely random motion. • Liquid particles appear to move around moving points. • Solid particles appear to move around fixed points. 3.Collisions are perfectly elastic (no change within the entire Kinetic Energy of two particles before and after their collision) ; No loss of energy! Kinetic energy (K.E.) is the energy of motion. It depends on the MASS of the thing and its VELOCITY (SPEED) Temperature = measure of K.E. (how fast molecules travel) Temperature is directly proportional with K.E. When the temperature decreases, the particle speed also decreases. (i.e., a decrease in kinetic energy) When the temperature increases, the particle speed rises too. (i.e., a rise in KE) Kelvin (K) = temperature scale used in Kinetic study ⇒ Absolute Zero temperature in which all molecular motion stops = (0 K or - 273◦ C) Conversion between ◦ C and K: K = ◦ C + 273 or ◦ C= K-273 Figure 2. Comparison of Temperature scales Source: https://www.sheffield.k12.oh.us Kinetic Theory Description of the Liquid State According to the kinetic theory of gases, liquid particles' motion is often described as a kind of matter that features a definite volume and takes the form of its container. 1. Particles in a liquid are in constant motion; however, the particles in a liquid are closer together than those in a gas. 2. Therefore, the attractive forces between particles in a liquid are stronger than those between particles in a gas. 3. This attraction is caused by intermolecular forces. 4. According to the kinetic-molecular theory of liquids, the particles aren't bound together in fixed positions. Kinetic-Theory Description of the Solid State According to the kinetic theory, solid particles' motion is often described as lower K.E., less motion, more packed particles, and stronger intermolecular forces (IMF). 1. Intermolecular forces between particles are therefore far more effective in solids. 2. These hold particles of a solid in relatively fixed positions, with only vibrational movement. 3. Solids are more ordered than liquids and gases. Table 1. Properties of Liquids and Solids: The Particle Model Property Liquids Solids Volume/Shape Definite volume but indefinite Definite volume and shape -solids shape- can't expand to fill a maintain a particular shape without a container; Volume is constant container because of closely packed particles Fluidity Density Compressibility Diffusibility Motion of Molecules Non-fluid- solid particles can’t flow because they are held in relatively fixed positions Relative high density- close High density- solids are packed more arrangement of particles closely than that of a liquid or gas. (compared to a gas) making mass/volume ratio higher Much less compressible than Incompressible - particles are packed gases because particles are so close together there’s virtually no space between them. closer together. Slower diffusion than gasesSlow diffusion – much slower than mix with other liquids due to liquids due to the high IMF’s between the constant motion of particles particles Random motion, medium Vibration in place speed, limited distances Fluid-ability to flow and take the form of the container Figure 3. Examples of Solids and Liquids Activity 1. Properties of Liquids and Solids! Learning Objective: Describe the characteristic properties that differentiate liquids and solids. What you need: pen and paper What to do: Complete the table below in comparing the properties of liquids and solids. Molecular Behavior Properties of Matter liquid solid Volume/shape Density Compressibility Motion of Molecules Guide Questions: 1. Differentiate the distances between molecules in the liquid and solid. 2. Rank the phases in increasing distance between particles. Activity 2. Kinetic Molecular Model of Liquids and Solids! Learning Objective: Apply the kinetic molecular theory to describe liquids and solids. What you need: pen and paper What to do: Part A. Illustrate the molecular representation of solid and liquid using any shape of your choice in the boxes below and briefly discuss each illustration. Use another sheet of paper for your answer. Solid Discussion: Liquid Discussion: Rubrics: 3 – Explanation is scientifically consistent with the concepts and has no misconception. 2 - Explanation is scientifically consistent with the concepts but with minimal misconception. 1 – Explanation is consistent with the concepts but with misconceptions. 0 - No discussion. Reflection Direction: Answer the question below in five sentences on a separate sheet of paper. An explosion aboard a power barge off the Philippine island of Guimaras has spilled up to a quarter million liters of fuel oil, threatening local communities, mangrove and seagrass habitats. Explain the effects of an oil spill in bodies of water by applying the concept of Kinetic molecular theory. Rubrics: 5 –Explanation is scientifically consistent with the concepts and has no misconception. 4 - Explanation is scientifically consistent with the concepts but with minimal misconception. 3 –Explanation is consistent with the concepts but with misconceptions. 2- Explanation is not consistent with the concepts. 0 - No discussion. References for learners: COHASSET PUBLIC SCHOOLS. “Kinetic Molecular Theory Worksheet”. Accessed January 12, 2021.https://www.cohassetk12.org/cms/lib010/MA01907530/Centricity/Domain/34 5/Intro%20Chem%20A/Unit%203%20-%20States%20of%20Matter/3-3%20KMT% CRSD.ORG.“Liquids and Solids” Accessed January 11, 2021. https://www.crsd.org/cms/lib/PA01000188/Centricity/Domain/676/Liquids and Solids.ppt Sheffield. “Kinetic Molecular Theory” Accessed January 12, 2021. https://www.sheffield.k12.oh.us/Downloads/KMT%20notes%20and%20WS.pdf Teacherph. “General Chemistry 2: Senior High School SHS Teaching Guide “Accessed January 9, 2021.https://www.teacherph.com/general-chemistry-2-teaching-guide/ West Linn-Wilsonville School District.”Kinetic Molecular Theory” Accessed January 12, 2021. https://www.wlwv.k12.or.us/cms/lib8/OR01001812/Centricity/Domain/1638/Unit%20 7%20HW%20Packet.pdf 2 3