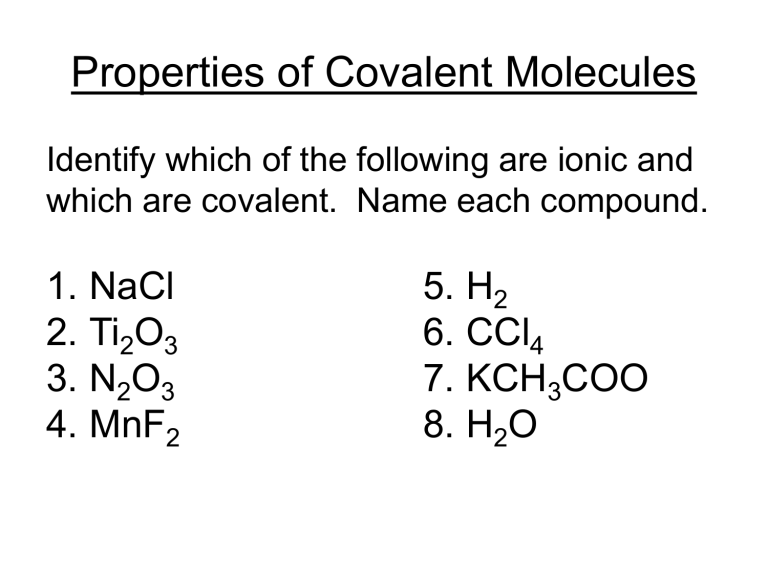
Properties of Covalent Molecules Identify which of the following are ionic and which are covalent. Name each compound. 1. NaCl 2. Ti2O3 3. N2O3 4. MnF2 5. H2 6. CCl4 7. KCH3COO 8. H2O Properties of Covalent Molecules Covalent molecules are generally small, made up of non-metals that are strongly bonded together. Covalent molecules are uncharged so many do not dissolve in water. They have low melting and boiling points because there are weak bonds between the molecules. There are weak bonds between the molecules so they are generally soft and spread easily. Covalent compounds are not charged and so cannot conduct heat or electricity. Covalent vs Ionic Compounds Covalent (molecular compound) Ionic


