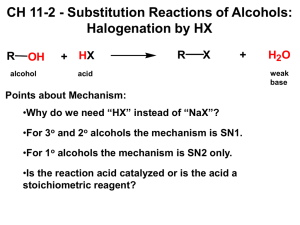Reactions of Alcohol, Ethers, Epoxides, Amines and Thiols Nomenclature of Alcohols A compound in which a hydrogen of an alkane has been replaced by an OH group Replace ‘e’ at the end of the parent hydrocarbon name with ‘ol’ 1. Identify longest chain containing the functional group. Number the chain in the direction that gives the functional group suffix the lowest possible number. 2. If there is a functional group suffix and a substituent, the functional group suffix gets the lowest possible number 3. If counting in either direction gives the same number for the functional group suffix, the chain is then numbered in the direction that gives a substituent the lowest possible number. a. Number is not needed to designate the position of a functional group suffix in a cyclic compound, because it is assumed to be at the 1-position 4. If there is more than one substituent, the substituents are stated in alphabetical order Activating an Alcohol for Nucleophilic Substitution by Protonation OH- is a strongly basic leaving group that cannot be displaced by a nucleophile To convert OH group into a weaker base, protonate it by adding acid to the reaction mixture → changes the leaving group from OH- to H2O, which is a weak enough base to be displaced by a nucleophile Substitution reaction is slow and requires heat for it to take place at a reasonable rate Only weakly basic nucleophiles (I-, Br-, Cl-) can be used in substitution reaction Primary, secondary and tertiary alcohols all undergo nucleophilic substitution reactions with HI, HBr and HCl to form alkyl halides Primary and secondary alcohols require heat for this reaction; Tertiary alcohols do not Mechanism for SN1 Reaction of an Alcohol Acid protonates the most basic atom in the molecule Weakly basic water is the leaving group that is expelled, forming a carbocation Carbocation can combine with a nucleophile and form a substitution product, or it can lose a proton and form an elimination product Little elimination product is actually obtained because the alkene formed in an elimination reaction can undergo a subsequent electrophilic addition reaction with HBr to form more of the substitution product Tertiary alcohols undergo substitution reaction with hydrogen halides faster than secondary alcohols do → tertiary carbocations are more stable → formed more rapidly → reaction proceeds readily at room temperature Reaction of a secondary alcohol with a hydrogen halide has to be heated to have the reaction occur at a reasonable rate Primary alcohols cannot undergo SN1 reactions because primary carbocations are too unstable to be formed, even when the reaction is heated Mechanism for SN2 Reaction of an Alcohol Primary alcohols undergo SN2 reactions with hydrogen halides Acid protonates the most basic atom in the reactant Nucleophile attacks the back side of the carbon and displaces the leaving group Only substitution product is obtained No elimination product is formed because halide ion, although a good nucleophile, is a weak base o A strong base is required to remove a hydrogen from a 𝛽-carbon in an E2 reaction Elimination Reaction of Alcohols: Dehydration