Quantum Mechanics: Photons, Photoelectric Effect, and Radiation
advertisement

Quantum Mechanics
In the early 1900s physicists performed experiments that probed reality at its fundamental level.
They found that matter was made of particles called atoms. Inside each atom they found the
subatomic particles: electrons, protons, and neutrons. They also found that light was made of
particles that we now call photons.
The strangest result was that these particles produced wave-like interference patterns. The
separate wave and particle models kjfbv accurate.
the particle model vs. the wave model
The overlap between the wave and particle models at the nanometer scale led to many more
exciting experiments. Over time, physicists developed and refined a new model, called quantum
mechanics. Predictions based on quantum mechanics can only give probabilities, but when
applied to repeated experiments they have an unparalleled level of accuracy.
An understanding of quantum mechanics is typically achieved after studying the underlying
math. Building an intuition without the math can be confusing, but here is a brief and inevitably
flawed description.
how quantum mechanics makes predictions
interpretations of quantum mechanics
wave-particle duality
quantum uncertainty
observing quantum phenomena
Quantum mechanics seems strange from our perspective. This feeling might come from the
expectation that our everyday experience of reality is normal, and quantum mechanics is for
special cases involving very small particles. But, it's the other way around. Quantum mechanics
describes the fundamental rules of reality, and our everyday experience is a special case of
quantum mechanics.
Photon Energy
In 1899, after investigating the thermal radiation spectrum, Maxwell Planck reluctantly
hypothesized that the energy of light is only released in small quantities determined by its
frequency. This idea was the beginning of quantum mechanics.
e-e-e-eThese packets of light were eventually called photons. Photons have a frequency and a
wavelength, but no mass. They are produced anytime a charged particle loses energy. Photons
spread out propagating through space at the speed of light. They can transfer their energy to a
charged particle in a process called absorption.
E=hfE=hfE=hf
EEE = energy of a photon [J, joules, kg m²/s²]
hhh = 6.626 × 10-34 = Planck's constant [J s]
fff = frequency [Hz, 1/s]
f (THz)
668 – 789
606 – 668
526 – 606
508 – 526
484 – 508
400 – 484
λ (nm)
380 – 450
450 – 495
495 – 570
570 – 590
590 – 620
620 – 750
Question: Which has more energy, one red photon or one blue photon? answer
Example: What is the energy range for red photons? Calculate the highest and lowest energy
possible in the color ranges listed above. metric prefixes solution
Example: The frequency of a photon is 3.6 × 1015 Hz. What color is it? What energy does it
have? electro-magnetic spectrum solution
Example: Find the energy for a photon that has a wavelength of 0.3 m. What part of the E-M
spectrum is the photon in? solution
Example: Find the wavelength of a photon that has 2.65 × 10-19 J of energy. solution
Example: A cheap helium neon laser outputs 5 mW of optical power. Lookup the wavelength of
the light and then calculate the number of photons produced every second. strategy solution
15 900 000 000 000 000 photons/second from a low power laser beam!
The Photoelectric Effect
The photoelectric effect occurs when metals dislodge electrons after being hit by light. Light
with a frequency above the visible spectrum is required to produce the effect. Bright red light
can't produce the effect, but even dim UV light can.
In 1905, Albert Einstein published an explanation of the photoelectric effect that supported Max
Plank's concept of quantized light. Einstein suggested that light is made up of many small
packets of energy, and each packet interacts with a single electron. Only high frequency light
releases electrons because it has enough energy per packet.
Einstein published an equation that describes the photoelectric effect using conservation of
energy. The kinetic energy of a released electron can't exceed the difference between the energy
of an incoming photon and the energy needed to dislodge the electron. If the maximum kinetic
energy is below zero, an electron is not released.
Kmax=hf−ΦK_{max}=hf - \PhiKmax=hf−Φ
KmaxK_{max}Kmax = maximum kinetic energy of released electron [J, joules]
hhh = 6.626 × 10-34 = Planck's constant [J s]
fff = frequency of incoming light [Hz, 1/s]
Φ\PhiΦ = Work function, the minimum energy to dislodge an electron [J]
Electrons can be thought of as being stuck in a energy well. The work function represents the
minimum energy the electrons needs to escape. The work function depends on the material.
Metals have a low work function, so it is easier to dislodge an electron from a metal.
Question: Why does dim ultraviolet light produce the photoelectric effect, yet very bright red
light doesn't? answer
Energy at the atomic level is often calculated in electron volts (eV). We can convert between eV
and J by multiplying or dividing by the charge of an electron.
1eV=1.6×10−19J1 \, \mathrm{eV} = 1.6 \times 10^{-19} \, \mathrm{J} 1eV=1.6×10−19J
32×10−19J(1eV1.6×10−19J)=20eV32 \times 10^{-19} \, \mathrm{J} \left( \frac{ 1 \,
\mathrm{eV}}{ 1.6 \times 10^{-19} \, \mathrm{J}} \right)= 20 \,
\mathrm{eV}32×10−19J(1.6×10−19J1eV)=20eV Example: Find the energy of a 1.69 × 1015 Hz
photon in electron-volts. solution
Example: After being hit by light with 7.0 eV per photon, the rare earth metal terbium releases
electrons. The electrons have a maximum kinetic energy of 4.0 eV. What is the work function of
terbium in electron-volts? solution
Example: Find the max kinetic energy of a magnesium electron after being hit by a photon with
a frequency of 600 THz. Look up the work function for magnesium and convert it into joules.
metric prefixes solution
Example: A 100 nm photon strikes a lump of magnesium. How much kinetic energy could the
released electron have? solution
How fast could the electron be moving as it escapes the magnesium atom? solution
Example: Use the work function table to decide which elements would release electrons from
427 nm wavelength light. strategy solution
Light as Radiation
Radiation is a wave or particle that transmits energy through space. This includes particles with
mass (electrons) and massless particles (photons).
A large amount of radiation can hurt living things in a direct way, by increasing their
temperature. A toaster and a microwave oven both use radiation to cook food.
Radiation from particles with very high energy can harm living things in more subtle way. The
particles can ionize atoms, which breaks chemical bonds. This can cause cell death and possibly
cancer.
Non-ionizing radiation is generally below 1.60 × 10-18 J (10 eV). This safe radiation doesn't
have enough energy to break chemical bonds.
Ionizing radiation is generally above 1.60 × 10-18 J (10 eV). This unsafe radiation can
potentially break chemical bonds.
region
gamma ray
x-ray
ultraviolet
visible light
infrared
microwave
radio wave
wavelength (m)
frequency (Hz)
energy (J)
energy (eV
2 × 10-11
1 × 10-8
4 × 10-7
7.5 × 10-7
1 × 10-2
1
1.5 × 1019
3 × 1016
7.5 × 1014
4 × 1014
3 × 1010
3 × 108
10 × 10-15
2.0 × 10-17
5.0 × 10-19
2.7 × 10-19
2.0 × 10-23
2.0 × 10-25
62 500
125
3.1
1.7
0.000 125
0.000 001
Click to Run
Try this PhET simulation to see how molecules interact with photons.
Question: Greenhouse gasses need to interact with infrared light. Which molecules from the
simulation could be greenhouse gasses? answer
Question: Can microwave ovens ionize atoms? Are microwaves dangerous? answer
Question: Is sunlight dangerous? answer
Question: Which would damage a person the most: an ultraviolet photon or a gamma-ray
photon? answer
Wavelength and Momentum
We can calculate the quantum wavelength of a particle in terms of it's momentum with the
equation below.
derivation of particle wavelengths
λ=hp\lambda = \frac{h}{p}λ=ph
λ\lambdaλ = wavelength [m, meter]
hhh = 6.626 × 10-34 = Planck's constant [J s]
ppp = momentum [kg m/s]
Photons have no mass, but they they still have momentum. You can actually push a space ship
like a sail boat, but with light instead of wind. When photons collide with the light sail, they
bounce off pushing the sail forward.
Example: What is the momentum of a photon of green light? (540 nm) solution How many
green photons would it take to accelerate a 1 kg body from rest to 1 m/s? (assume the green
photons reflect off the 1 kg object)
In 1924 Louis de Broglie proposed that particles with mass might have a wavelength similar to a
photon's. This turned out to be accurate. Electrons, atoms, even large molecules all have a
measurable quantum wavelength based on their momentum.
λ=hpλ=hmv\lambda = \frac{h}{p} \quad \quad \lambda = \frac{h}{mv}λ=phλ=mvh
particle
mass (kg) speed (m/s) wavelength (m)
radio photon 0
c
≈1
-31
electron
9.1 × 10 1
≈ 10-4
visible photon 0
c
≈ 10-7
oxygen atom 2.7 × 10-26 1
≈ 10-8
gamma ray 0
c
≈ 10-12
electron
9.1 × 10-31 0.5c
≈ 10-12
cat
4
1
≈ 10-36
Particles the size of a cat have extremely small wavelengths, but cats aren't exactly a single
quantum particle. The equation begins to lose meaning at the macroscopic scale. This agrees
with our everyday observations for cats. They don't have a measurable wave-nature, although
they are very sneaky.
Example: Find the wavelength of a proton moving at 30 m/s. Subatomic Particles Data Table
solution
Emission and Absorption
Each electron in an atom can only have an energy that exactly matches an atomic orbital.
quantum mechanics and atomic orbitals
If an electron gains the energy difference between two states it can jump to a higher energy level.
For example, an electron will transition to a higher energy level if it collides with a photon that
has an energy equal to the energy difference between levels.
This process can also run in reverse. An electron can drop to a lower energy level if an an atom
has an unoccupied energy level. When the electron drops down it emits a photon equal to the
difference in energy between each level. This follows the law of conservation of energy.
Photon emission occurs when electrons transition to lower energy levels within an atom. Each
electron transition emits a photon with an energy equal to the energy difference between levels.
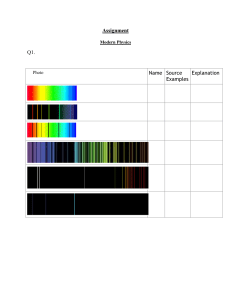
Hydrogen has a very simple emission spectrum because it doesn't have very many possible
energy states and therefore few energy state transitions.
Iron's nucleus has more protons so it has more possible energy transitions.
Photon emission is the working principle behind fluorescent lights. To make light, a tube is filled
with various gases. The gases are electrically charged up which brings the electrons to a higher
energy level. The electrons are unstable in the higher energy levels. They eventually fall back
down to their ground state and emit light. Try looking at fluorescent light reflected off a CD to
see the separate bands of color.
Example: Find the frequency of the photon produced when an electron drops from an energy of
-3.4 eV to -13.6 eV in a hydrogen atom? solution
Absorption is the reverse of emission. A single photon is absorbed by a single electron in a
single atom. This causes the electron to transition to higher energy levels.
If there isn't an energy difference that matches the energy of the colliding photon, the material is
transparent to that frequency. A substance may be clear in one range of the spectrum but not in
others. For example: glass is mostly transparent to visible light, but it has many absorption
frequencies in the infrared.
Exposing an atom to a full range of light will produce an absorption spectrum that matches the
energy difference between electron energy levels.
Light absorption occurs at the same energies as emission. Hydrogen's absorption frequencies are
the same as its emission frequencies.
Analyzing the spectrum of emission or absorption can actually be used like a fingerprint to
identify the elements or molecules being observed. This technique is used in fields like forensics
and astronomy.
Sunlight is mostly thermal radiation with large sections of absorption from the molecules in
Earth's atmosphere.
250 500 750 1000 1250 1500 1750 2000 2250 2500 Wavelength (nm) Spectrum of Solar
Radiation (Earth) 0 0.5 1 1.5 2 2.5 Irradiance (W/m²/nm)
2 H O Atmospheric absorption bands H O 2 H O 2 H O 2 H O 2 CO 2 O 2 O 3 UV Visible
Infrared Sunlight without atmospheric absorption 5778K blackbody Sunlight at sea level
Question: What molecules are absorbing most of the Sun's infrared rays? answer
Question: What molecule is responsible for absorbing the Sun's UV rays? answer
103 nm n = 1 n = 2 n = 3 n = 4 n = 6 n = 5 434 nm 122 nm Lyman series Balmer series Paschen
series 94 nm 410 nm 486 nm 656 nm 1875 nm 1282 nm 1094 nm 97 nm 95 nm
This diagram shows the wavelengths of photons emitted or absorbed when an electron transitions
between energy levels. Each wavelength, listed in nanometers, is for a photon with an energy
equal to the difference between energy levels.
Question: What wavelength of light could make an electron jump from energy level n = 1 to n =
5? answer
Example: Find the energy of a photon produced as an electron drops from energy level n = 4 to
n = 2? solution
Example: Find three possible light frequencies that could be absorbed by an electron at energy
level n = 3. solution
Back