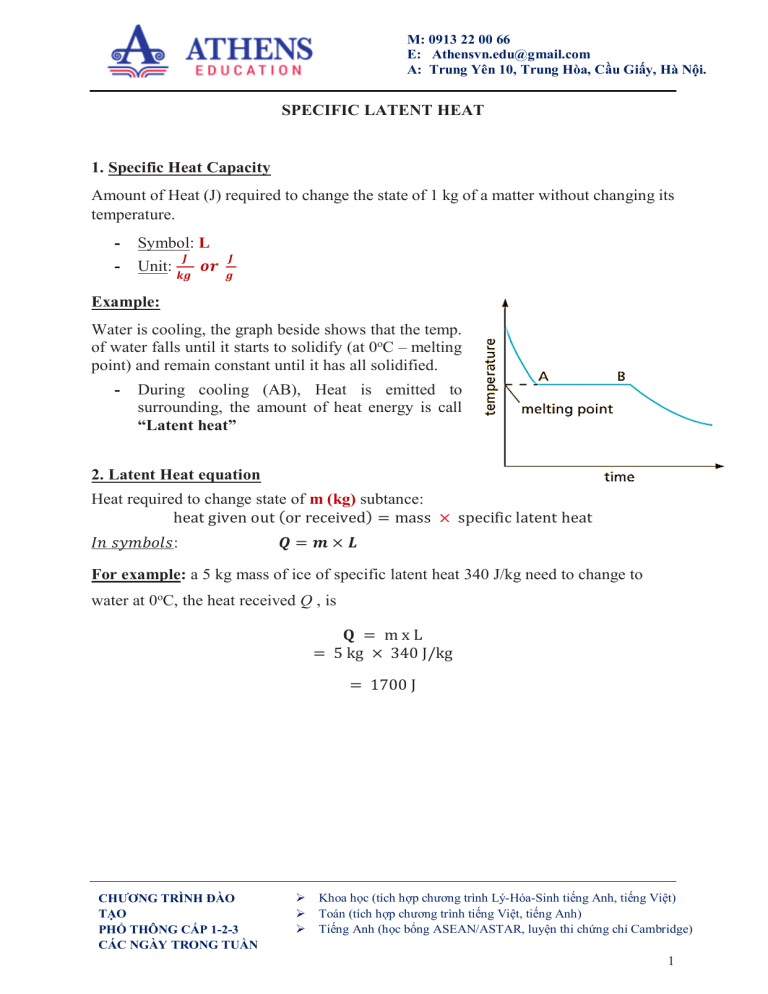
M: 0913 22 00 66 E: Athensvn.edu@gmail.com A: Trung Yên 10, Trung Hòa, Cầu Giấy, Hà Nội. SPECIFIC LATENT HEAT 1. Specific Heat Capacity Amount of Heat (J) required to change the state of 1 kg of a matter without changing its temperature. - Symbol: L - Unit: 𝑱 𝒌𝒈 𝒐𝒓 𝑱 𝒈 Example: Water is cooling, the graph beside shows that the temp. of water falls until it starts to solidify (at 0oC – melting point) and remain constant until it has all solidified. - During cooling (AB), Heat is emitted to surrounding, the amount of heat energy is call “Latent heat” 2. Latent Heat equation Heat required to change state of m (kg) subtance: heat given out (or received) = mass × specific latent heat 𝐼𝑛 𝑠𝑦𝑚𝑏𝑜𝑙𝑠: 𝑸= 𝒎×𝑳 For example: a 5 kg mass of ice of specific latent heat 340 J/kg need to change to water at 0oC, the heat received Q , is 𝐐 = mxL = 5 kg × 340 J/kg = 1700 J CHƯƠNG TRÌNH ĐÀO TẠO PHỔ THÔNG CẤP 1-2-3 CÁC NGÀY TRONG TUẦN Khoa học (tích hợp chương trình Lý-Hóa-Sinh tiếng Anh, tiếng Việt) Toán (tích hợp chương trình tiếng Việt, tiếng Anh) Tiếng Anh (học bổng ASEAN/ASTAR, luyện thi chứng chỉ Cambridge) 1 M: 0913 22 00 66 E: Athensvn.edu@gmail.com A: Trung Yên 10, Trung Hòa, Cầu Giấy, Hà Nội. Practice exercises 1. a How much heat will change 10 g of ice at 0 ºC to water at 0 ºC? b What quantity of heat must be removed from 20 g of water at 0 ºC to change it to ice at 0 ºC? 2. a How much heat is needed to change 5 g of ice at 0 ºC to water at 50 ºC? b If freezing a freezer point cools in 10 200 g of minutes, water how from much 20 heat ºC is to its removed per minute from the water? 3. How long will it take a 50 W heater to melt 100 g of ice at 0 ºC ? (Known that 𝑃𝑜𝑤𝑒𝑟 = 𝐸𝑛𝑒𝑟𝑔𝑦 / 𝑡𝑖𝑚𝑒 𝑖𝑛𝑡𝑒𝑟𝑣𝑎𝑙 ) 4. Some small aluminium rivets of total mass 170 g and at 100 ºC are emptied into a hole in a large block of ice at 0 ºC. a What will be the fi nal temperature of the rivets? b How much ice will melt? 5. The values in Table below are required. a) How much heat is needed to change 20 g of ice at 0 ºC to steam at 100 ºC? b) An aluminium can of mass 100 g contains 200 g of water. Both, initially at 15 ºC, are placed in a freezer at -5.0 ºC. Calculate the quantity of heat that has to be removed from the water and the can for their temperatures to fall to -5.0 ºC. CHƯƠNG TRÌNH ĐÀO TẠO PHỔ THÔNG CẤP 1-2-3 CÁC NGÀY TRONG TUẦN Khoa học (tích hợp chương trình Lý-Hóa-Sinh tiếng Anh, tiếng Việt) Toán (tích hợp chương trình tiếng Việt, tiếng Anh) Tiếng Anh (học bổng ASEAN/ASTAR, luyện thi chứng chỉ Cambridge) 2