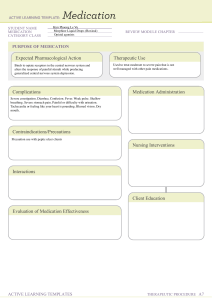
DISPENSING ALL LECTURES - for Review in Medication Comprehensive
advertisement

DISPENSING II LECTURE NOTES I. Prescription Analysis which stands for the word recipe (meaning, in Latin, to take); the inscription, which contains the names and quantities of the ingredients; the subscription or directions for compounding the drug; and the signature which is often preceded by the sign "s" standing for signa (Latin for mark), giving the directions to be marked on the container. (typically used for OUTPATIENT care) MEDICATION ORDER: written directions provided by a prescribing practitioner for a specific medication to be administered to an individual. The prescribing practitioner may also give a medication order verbally to a licensed person such as a pharmacist or a nurse. (typically used for IN-PATIENT care) Notice how complicated this one goes? With it, how do we make sure that we are accurately dispensing the medicine? Medication and Prescription Orders Study the descriptions and videos below, then answer the quiz found below. (see 'DISP 2 QUIZ' document below.) Though discouraged due to increasing medication safety issues, medical abbreviations are still in use in today's medical practice. As pharmacists, we have to be proficient in understanding these to ensure patient safety. A comprehensive list of medical abbreviations often encountered in prescription and medication orders are provided in the link. https://www.drugs.com/article/prescriptionabbreviations.html#abbrtable PRESCRIPTION: A physician's order for the preparation and administration of a drug or device for a patient. A prescription has several parts. They include the superscription or heading with the symbol "R" or "Rx", Common Medical Abbreviations Abbreviation 1/2NS Meaning / Intended Meaning one-half normal saline (0.45%) 5-ASA 5-aminosalicylic acid a A.M. aa AAA AAA before morning of each abdominal aortic aneurysm (called a "triple-A") apply to affected area ac achs AD ad lib ad sat. ad. before meals before meals and at bedtime right ear freely; as much as desired to saturation to; up to ALT alt. alt. h. am, A.M. amp amt. ant. ante ap APAP aPTT AQ, aq a.s., AS ASA AST ATC AU AZT alanine aminotransferase alternate every other hour in the morning; before noon ampule amount anterior before before dinner acetaminophen activated partial thromboplastin water left ear aspirin aspartate aminotransferase around the clock each ear; both ears zidovudine Notes About Confusion Normal saline (NS) is 0.9%, so one-half normal saline is 0.45% Can be misinterpreted as five tablets of aspirin (per FDA). Spell out full drug name. Can be misinterpreted as 'apply to affected area' Can be misinterpreted as 'abdominal aortic aneurysm' Caution not to confuse with AD (meaning right ear) Spell out drug name "acetaminophen" Spell out drug name "aspirin" Can be misinterpreted as azathioprine (per FDA). Spell out drug name. Ba BCP Bi barium birth control pills bismuth bid, BID BM BMI bol BP BPH BS BSA BT twice a day bowel movement body mass index bolus blood pressure benign prostatic hypertrophy blood sugar body surface area bedtime c C.C. c/o C&S CABG CaCO3 CAD CAP cap. with chief complaint complaints of culture and sensitivity coronary artery bypass graft calcium carbonate coronary artery disease cancer of the prostate capsule CBC cc CD CF cm CNS conc CPZ complete blood count cubic centimeters controlled delivery cystic fibrosis centimeter central nervous system concentrated Compazine CR cr, crm CV CXR D/C, dc, disc. controlled-release cream cardiovascular chest x-ray discontinue OR discharge D5/0.9 NaCl 5% dextrose and normal saline solution (0.9% NaCl) 5% dextrose and half normal saline solution (0.45% NaCl) dextrose 5% in normal saline (0.9%) 5% dextrose in water dispense as written diastolic blood pressure diluted dispense In U.S., 'hs' or 'HS' is more commonly used for bedtime. Do not confuse with "capsule" Do not confuse with "cancer of the prostate" Can be misinterpreted as chlorpromazine (per FDA). Spell out drug name. D5 1/2/NS D5NS D5W DAW DBP dil. disp Multiple possible meanings; spell out instead of using "D/C" div DKA dL DM DO DOB DPT divide diabetic ketoacidosis deciliter diabetes mellitus Doctor of Osteopathic Medicine date of birth diphtheria-pertussis-tetanus DR DVT DW EC EENT elix. emuls. ER ER ETOH F f or F FBS FDA Fe FFP fl or fld ft G, or g, or gm garg GERD GI gr. delayed-release deep vein thrombosis dextrose in water, diabetes mellitus or distilled water enteric-coated Eye, Ear, Nose, and Throat elixir emulsion extended-release emergency room ethyl alcohol Fahrenheit female fasting blood sugar Food and Drug Administration Iron fresh frozen plasma fluid foot gram gargle gastroesophageal reflux disease gastrointestinal grain GTT gtt, gtts glucose tolerance test drop, drops GU guttat. h, or hr. h/o H&H H2 H20 HAART HCT, or Hct genitourinary drop by drop hour history of hematocrit and hemoglobin histamine 2 water highly active antiretroviral therapy hematocrit Better to spell out vaccine name; can be misinterpreted as Demerol-PhenerganThorazine per FDA Multiple possible meanings; spell out instead of using "DW" Can also mean "emergency room" Can also mean "extended-release" "g" is preferred symbol Apothecary measurement (obsolete and may be misinterpreted as gram; do not use) Can be confused with gtt for drops Can be confused with GTT for glucose tolerance test HCT hydrocortisone HCTZ hydrochlorothiazide HR HS heart rate half-strength hs or HS HTN hx IBW ID at bedtime, hours of sleep hypertension history ideal body weight intradermal OR infectious disease IJ IM IN inf inj. instill. IP IR injection intramuscular intranasal infusion injection instillation intraperitoneal immediate-release international unit(s) IU IUD IV IVP intrauterine device intravenous intravenous push IVPB J K KOH L or l intravenous piggyback joule potassium potassium hydroxide liter LA lab lb. LDL LFT Li liq. long-acting laboratory pound low-density lipoprotein liver function tests lithium liquid Better to spell out drug name; can be misinterpreted as hydrochlorothiazide per FDA Better to spell out drug name; can be misinterpreted as hydrocortisone per FDA better to spell out; do not mistake for "bedtime" Do not misinterpret as 'half-strength' Multiple possible meanings; spell out word instead of using "ID" better to spell out 'injection' Mistaken as IV (intravenous) or the number 10 (ten); Instead use "International Unit(s)" (per Joint Commission's "Do Not Use" List of Abbreviations) Could be confused with 'intravenous pyelogram' Lowercase letter l may be mistaken as the number 1 (per ISMP). Instead use L (uppercase) for liter. LMP lot LPN LR mane mcg or µg last menstrual period lotion licensed practical nurse lactated ringer (solution) in the morning microgram MD MDI mEq mEq/L Mg mg MgSO4 medical doctor metered-dose inhaler milliequivalent milliequivalent per liter magnesium milligram magnesium sulfate mL milliliter mm MM or M M or K mm of Hg mMol MMR mol wt MR MS millimeter million thousand millimeters of mercury millimole measle-mumps-rubella (vaccine) molecular weight modified-release morphine sulfate or magnesium sulfate MSO4 morphine sulfate n or noct. N/A N/V, N&V Na NAS NDC Ng or ng in the night not applicable nausea and vomiting sodium intranasal National Drug Code nanogram NGT NH3 nasogastric tube ammonia Can be misinterpreted to mean "mg" or milligram, better to spell out 'microgram' May be confused with "MSO4" (morphine sulfate), spell out "magnesium sulfate" Joint Commission's "Do Not Use" List of Abbreviations Do not use ml as lowercase l may be mistaken for the number 1. Use mL (lowercase m, uppercase L) for milliliter (per ISMP). May be mistaken as thousand. Use million. May be mistaken as million. Use thousand. Can mean either morphine sulfate or magnesium sulfate, spell out full drug name - Joint Commission's "Do Not Use" List of Abbreviations May be confused with "MgSO4"; instead spell out "morphine sulfate" - Joint Commission's "Do Not Use" List of Abbreviations May be mistaken as mg or nasogastric. Use nanogram. NKA NKDA noct. maneq. NP NPO, n.p.o. no known allergies no known drug allergies night and morning nurse practitioner nothing by mouth NS NSAID NTE O2 OC o.d., OD normal saline nonsteroidal anti-inflammatory drug not to exceed oxygen oral contraceptive right eye o.d. once per day OJ o.s., OS OTC PA pc PRN PM PO orange juice left eye over-the-counter Physician Assistant after meals as needed evening orally or by mouth q q4h q6h q8h q12h qam qd, QD, q.d., Q.D. every every 4 hours every 6 hours every 8 hours every 12 hours every morning every day qhs each night at bedtime q.i.d. , QID qod, QOD, q.o.d., or Q.O.D. four times a day every other day Preferred by AMA to spell out "nothing by mouth" Can also mean "overdose" or "once daily"; better to spell out "right eye" Preferred in the UK; Can also mean "overdose" or "right eye"; better to spell out "once per day" May be better to spell out "by mouth" or "orally" (per AMA) Can be mistaken as q.i.d. Instead write "daily" (per The Joint Commission "Do Not Use List") or "use daily" per ISMP list Can be confused with "qh" (every hour); better to spell out "each night at bedtime" May be mistaken as qid or QID (four times daily). Write "every other day" (per ISMP and The Joint Commission). RA Rx SA SL, s.l. rheumatoid arthritis prescription sustained action sublingual (under the tongue) SC, SQ sq, or sub q subcutaneous or subcutaneously SR STD supp susp syr T tbsp or Tbsp sustained release sexually transmitted disease suppository suspension syrup temperature tablespoon TID, t.i.d. top. TR tsp three times a day topical timed-release teaspoon U or u unit ud, ut, dict, UD ung UTI WBC XR mcg, µg as directed ointment urinary tract infection white blood cell extended-release microgram Use SUBQ (all uppercase) or spell out subcutaneous or subcutaneously Mistaken as teaspoon(s). Use the metric system (e.g., mL). Mistaken as tablespoon(s). Use the metric system (e.g., mL). Mistaken as the number "0" (zero), the number "4" (four) or as "cc". Write "unit" instead (per The Joint Commission "Do Not Use" List). µg mcg can be misinterpreted as "mg". Better to spell out "microgram" To know how to read and understand prescription and medication orders, a series of explanatory videos are provided below. For professionals: 1. How to read prescription orders: READING PRESCRIPTIONS What are Sig Codes? ▪ ▪ Sig codes are shorthand for medical instructions From “signetur” (latin) – let it be marked or labeled Specific Forms ▪ Tablet ▪ Capsule ▪ Otic/ophthalmic Drops ▪ Creams ▪ Ointment ▪ Suspensions ▪ Injection ▪ Suppository Where on Body to Take? How Often to Take? ▪ q – Every ▪ qH – Every Hour ▪ qAM – Every Morning ▪ qPM – Every Evening ▪ qHS – Every Bedtime ▪ qD – Every Day ▪ qOD – Every Other Day ▪ qWK – Every Week ▪ BID – Two Times a Day ▪ TID – Three Times a Day ▪ QID – Four Times a Day ▪ PRN – If Needed When To Take During the Day? ▪ AC – Before a Meal ▪ PC – After a Meal ▪ HS – At Bedtime ▪ AM – Morning ▪ PM – Evening ▪ C – With ▪ OD – Right Eye ▪ OS – Left Eye ▪ OU – Both Eyes ▪ AD – Right Ear ▪ AS – Left Ear ▪ AU – Both Ears ▪ PO – By Mouth ▪ PR – Rectally ▪ PV – Vaginally ▪ IM – Intramuscular ▪ Buccal – Cheek/gum Other Things to Look For ▪ Quantity/Dose ▪ Variant delivery o SR – sustained release o XR – extended release ▪ Refills ▪ Generic or Brand Wrapping Up ▪ If you work in the pharmacy, its always good to double check your dispensing exactly as prescribed. ▪ Doctors makes mistakes to though, and sometimes a pharmacist may need to verify if something seems off or some information is not included. ▪ If you’re a patient, its also good to verify your medication as well. Knowing what you’re taking is always a smart choice. You can always ask for explanation of instructions and the purpose of medication too before leaving the prescribers. ▪ Educating yourself about prescriptions is a step in the right direction. ▪ Medications are beneficial when taken correctly, but people can always make mistakes which is why everyone should do their best to make sure that doesn’t happen, and the patient continues on a healthier path. Standard written order - if a standard written order doesn’t specify a time period, then it usually remains in effect until it’s discontinued. Single order 2. How to read medication orders: When a drug should be given only once, a prescriber writes what’s called a single order. INTERPRETING MEDICATION ORDERS In the hospital, a prescriber typically orders a drug by writing instructions on an order sheet in the patient’s chart. In some facilities, the prescriber enters the order in the computer. Either way however, you may fine one of several different types of medication orders. Types of medication orders • Standard written orders • Single orders • Stat orders • P.R.N. orders • Verbal orders Stat order - if a patient needs a medication right away for an urgent problem, the prescriber will write a stat order. P.R.N. order - - The form and amount of drug to give - The administration route - The time schedule for administration - The prescriber’s signature The term P.R.N. comes from a Latin phrase that means as the occasion arises. Thus, a P.R.N. order allows you to give a medication when the patient needs it. Sometimes a P.R.N. order specifies a reason for giving the drug. Verbal order - Is one that a prescriber tells you in person or over the phone, rather writing it down. - Verbal orders are easier to interpret than written order. So, you should avoid them whenever possible. If you must take a verbal order, repeat it clearly back to the prescriber and ask the prescriber to sign your copy as soon as possible. Regardless of the type of medication order a prescriber writes, it must include certain information, including: - Several types of medication errors can result form the orders themselves. Errors caused by medication orders • Poor handwriting • Incomplete orders • Dangerous abbreviations • Misplaced decimal points The patient’s full name Examples: Illegible handwriting - The date and time - - The name of the drug 5 mg or 3 mg? DOCTOR’S ORDERS – video *Notes written on lecture notebook* - Should be given orally, intramuscularly, or intravenously? And how often you should give it? Dangerous abbreviation - Should you give 10 or 100 units of subcutaneous insulin before each meal? Misplaced decimal point - This order says to give 1.25 milligrams of digoxin each day by the oral route, but is that within the acceptable range? Or did the prescriber really mean 0.125 milligrams? Any time you think an order is unclear or maybe incorrect, it’s your duty to clarify the order with the prescriber, the pharmacist, or your supervisor, before you give the drug to a patient. For patients: 3. Pharmacy’s phone number 1. How to read a prescription label: LET’S TALK ABOUT MEDICINES: PRESCRIPTION MEDICINE LABELS Its important to understand your medicine and to take it correctly. This starts with listening to your doctor, your pharmacist, and reading the prescription label. There is a lot of information on a prescription label, it’s important to know the main parts of the label to help keep you safe and to get most benefit out of your medicine. - 1. Your Name and Address You can call this number with questions for your pharmacist or to order a refill. 4. Your doctor’s name - This tells you that the prescription is for you and no one else. By law, you should not take certain medicines prescribed for another person, but more importantly it’s a risk to your health and safety to take someone else’s medicine. - This is the name of the doctor who prescribed this particular medicine. - Notice that your doctor’s phone number is not on the label, but the pharmacy phone number is. 2. Name and address of pharmacy - This let’s you know where you got the medicine and where it can be refilled. 5. Prescription date 7. The quantity - The date your pharmacy got your medicine ready for you 6. Use before date - Sometimes this is called the expiration date. You should not take the medicine after this date. It may not work as well as it should, and it may be harmful to do so. This is the number of tablets or capsules in the bottle on the day the prescription is filed. If your medicine is a liquid, the quantity may be measured in milliliters. 8. Name & strength of the medicine - This may be a brand name or a generic name or both. In this example, the label shows the drug naproxen in 500 mg tablets. 9. How much and how often 11. Prescription number - - How much medicine to take and how often you should take it. On this label, the direction read take one tablet by mouth every 12 hours. If you call your pharmacy for a refill, it is helpful to give them this number. 12. Scan code 10. Number of refills - The number of refills your doctor has approved. This tells you and the pharmacy how many times you my refill this prescription without contacting your doctor. - The code used to scan for refills. You can use this scan code to reorder your prescription. 2. How to teach your patients' parents in reading their child's prescription ▪ - ▪ ▪ The medication that is used and the concentration or form ▪ Quantity Prescription number It will help identify that prescription for your child Directions What ca be confusing for parents is understanding the difference between milliliters and milligram, so they’re both going to be units of measurement. The milliliters are going to be a unit measuring volume or the amount of liquid that you’re actually going to draw to give your child. Whereas, milligrams is going to be the actual does in weight of that medication that you’re receiving. - The most important part where parents should be focused more. The reason that the concentration is on here and why that is so important is because different medications have different concentrations. If we only know the amount of volume that you’re giving, we can’t determine what that actual dose is without understanding what this concentration is. They can vary from medication to medication. So, understanding both of these parts and having both of these on the prescription is really key to knowing the dose that your child is getting. It they’re admitted to the hospital or if you need to your doctor what they’re receiving. Both of those parts are crucial. - The quantity part, since it is liquid, there are 200 milliliters total in this bottle. After going through the labels, the really important part here is the volume piece, the 4 and a half ml, which your child’s dose that you want to administer. - It is really important to know how much volume that you need to draw up. So, you’re giving the right dose. If you’re confused and that dose is maybe in milligrams as opposed to milliliters. Understand that they’re not the same and if you have questions about that, you can always reach out to your pharmacist or to your pediatrician. Refer to Chapter 4 of your Pharmacy Calculations textbook on pp. 52-68 for a review of the concepts of Prescription and Medication Order Interpretations. MEDICATION REVIEW - Medication review is a structured evaluation of patient‘s medicines with the aim of optimizing medicines use and improving health outcomes. This entails detecting drug related problems and recommending interventions. Issues identified during a CMR should be resolved collaboratively with the patient and/or the patient’s care giver and the prescriber. - 3 sections in CMR: Comprehensive Medication Review o Preparation o Delivery o Documentation What is a Comprehensive Medication Review (CMR)? BEFORE THE CMR: (Video Lecture Notes): - - Medication therapy management is a term that has become increasingly well-known since the passing of the Medicare Modernization Act 2003. Pharmacists continue to be the leaders in providing MTM services which according to the MMA are designed to: o Enhance enrollee understanding of their medications o Improve adherence o Detect adverse drug events o Patterns of overuse and underuse related to their prescription medications - First step: Identify your CMR-targeted patients. o This can be done in the outcomes system. - Once you have your patients selected the next step is to contact the patient and offer the CMR service. This contact could be made by phone call or could be done face-to-face at the patient’s next visit to the pharmacy. - Many of the highest performing MTM centers empower technicians to identify CMR eligible patients and assist in making CMR offer. - Prior to the CMR appointment, print off a copy of the patient’s medication list, allergies, and disease conditions that are available to you. - Using available pharmacy resources, scan medication list for potential drug-drug interactions, drug-disease interactions, therapeutic duplication, compliance issues, cost-saving opportunities, and any other medication related problems that jump out of you. - Various services fall under the definition of MTM and each is important in optimizing patient care. One of the services that can be delivered as part of an MTM program is the COMPREHENSIVE MEDICATION REVIEW (CMR). - CMR is an appointment-based review of a patient’s prescription and over-the-counter medications by a pharmacist to identify medication related issues or cost-saving opportunities. - Be sure to review pending tips and drug therapy alerts in the outcome system. This will prepare you form the meeting. The focus of the CMR is: - Finally, be sure to have a CMR worksheet available. - o Medication safety o Patient compliance o Cost reduction o Creation of an medication list updated master DURING THE CMR: - The focus of the meeting is to identify all the medications that the patient is taking. - Talk about possible medication-related issues or cost-saving opportunities. After, a copy of updated medication list should be sent to the patient. - There are few things to keep in mind when conducting this appointment that will enhance patient participation: o To make a patient feel welcome and comfortable during the interview be conscious of your body language o Try to practice reflective listening. This is a strategy that involves listening to what the patient is saying and then communicating the message back to them in your own words to confirm that you understood their concern. This also allows you to communicate back to the patient what you perceive they’re feeling. o Another best practice to incorporate is using open ended questions. Using open ended questions will encourage the patient to share information but they may not have otherwise disclosed to you. AFTER CMR: - After the CMR, look over the information gathered: o Identify any medication related issues o Update the patient’s master medication list ▪ - and their MTM profile based on any new information or medications the patient’s might have brought to the appointment. Any problems identified or tips resolved can be build as additional encounters to the CMR. This would include any prescriber consultations that can result in changes in therapy, patient compliance consultations or OTC recommendations. - The pharmacist identified compliance intervention with the patient’s drug therapy - The pharmacist educated the patient on the importance of taking the drug as prescribed. - When prescriber consultation is necessary, an optional prescriber communication fax form is available. So, on the outcome system for use in communicating recommendations you feel appropriate. - Once you and the prescriber have agreed on any medication changes, notify the patient ang update the patient’s medication list. - The MTM profile is a tool that can be used to create a patient-friendly master medication list and medication action plan or map. This list and the map should then be mailed to the patient or picked up at their next visit. 4 out of 5 adults taking some sort of medication daily and the high cost of non-compliance to the healthcare system, the value of a CMR should be clear. Remember the CMR should help compile an up-to-date medication list, including over-the-counter meds which can be shared with the patient and their prescribers. It should also help identify any issues from a compliance safety and cost savings perspective that may involve some follow-up with the prescriber and finally, it will demonstrate the role of a pharmacist play in promoting the safe and effective use of medications to plan sponsors, prescribers, and patients. Patient barriers to CMR - Barrier #1: I already do this with my doctor - Barrier #2: I really don’t have time to do this - Barrier #3: I don’t have any questions COMPREHENSIVE MEDICATION REVIEW (CMR) 3 things to print off prior to a CMR appointment (Sir Sam’s ppt) ▪ Medication list - ▪ Allergies ▪ Disease conditions An essential component of medication therapy management What is Medication Therapy Management For? Things to scan for in the medication list prior to a CMR appointment ▪ Enhance enrollee understanding ▪ Improve Adherence ▪ Potential drug-drug interactions ▪ Detect Adverse Drug events ▪ Drug disease interactions ▪ Identify and intervene with overuse and underuse of medications ▪ Therapeutic duplication ▪ Compliance issues ▪ Cost saving opportunities What is Comprehensive Medication Review (CMR)? - Comprehensive Medication Review is an appointment-based review of a patient’s prescription and over the counter medications by a pharmacist to identify medication related issues or cost-saving opportunities. CMR Workflow Classifications of medicine reviewed under CMR ▪ Over-the-counter medicines, including herbal supplements ▪ Prescription drugs Focuses of CMR ▪ Medication safety ▪ Patient compliance ▪ Cost reduction ▪ Master medication list 3 sections of CMR ▪ Preparation ▪ Delivery ▪ Documentation Strategies to employ to make a patient feel comfortable during a CMR appointment ▪ Be conscious of your body language ▪ Try to practice reflective listening ▪ Using Open-ended questions Two activities completed after a successful CMR ▪ Identify any medication related issues. ▪ Update the patient’s master medication list. The Value of CMR ▪ The CMR should help compile an up-to-date medication list including over-the-counter meds which can be shared with the patient and their prescribers. ▪ Help identify any issues from a compliance safety and cost savings perspective that may involve some follow-up with the prescriber. ▪ It will demonstrate the role as a pharmacist play in promoting the safe and effective use of medications to plan sponsors, prescribers and patients 3. Subtherapeutic Dosage 4. Failure to Receive Drugs 5. Overdosage 6. Inverse Drug Reaction 7. Drug Interaction 8. Drug Use Without Indication Patient is being treated with an inadequate dose of the correct medication Patient has a medical problem that is the result of not receiving a drug Patient is being treated with too much of the correct drug. Patient has medical problem that is the result of an unintended and detrimental adverse drug effect. Patient has medical problem that is the result of a drug-drug, drugfood, or drug-laboratory interaction. Patient is taking a drug without a valid medical reason. MEDICATION-RELATED PROBLEMS A Medication-Related Problem (MRP), also referred to as a Drug Therapy Problem (DTP) is any undesirable event experienced by a patient that involves, or is suspected to involve, drug therapy, and that interferes with achieving the desired goals of therapy and requires professional judgment to resolve. PHARMACEUTICAL CARE DRUG THERAPY PROBLEMS Background Collection of S & O Information • If not resolved, drug therapy problems have clinical consequences. As clinical patient problems, drug therapy problems require professional (clinical) judgment to resolve. • Types and Definitions of MRP are found below. Type of MRP 1. Untreated Indication 2. Improper Drug Selection Definition Patient requires drug therapy but is not receiving medication for that indication. Patient requires drug therapy but is taking the wrong medication Data: - CC, HPI, PMH, PSHx, Demographics - Medication history compliance etc. - VS, ROS, Lab, other diagnostics Sources: - Patient, family - Other healthcare providers - Medical record/computer Background Assessment including • o In each medication: - Indicated? - Effective? - Safe? - One the patient will be compliant with? “Mr. M’s elbow pain is not being effectively controlled because the dosage of ketoprofen he has been taking for the past 3 days is too low” “My patient is experiencing orthostatic hypotension with mild to light headaches each morning because the 2 mg dose of risperidone she takes in the morning is too high” Drug Therapy Problems (DTP) - Undesirable event experienced by the patient which involves, or is suspected to involve drug therapy, and that interferes with achieving the desired goals of therapy. Need addition or modification of therapy to resolve or prevent undesirable event. DTP Categories 1. Drug therapy is unnecessary 2. Drug therapy needs to be added Components of DTP • Undesirable event or risk of event • Drug therapy involved • Relationship between undesirable event & drug 3. Drug not effective/producing desired response 4. Dosage too low to produce desired response 5. Drug is causing an adverse reaction 6. Dosage is too high resulting in toxicity 7. Patient not able/willing to take drug therapy Components of a DTP – details • Undesirable event: o o Medical complaint, sign, symptom, diagnosis, disease, illness, impairment, disability, abnormal laboratory value or syndrome. Can be result of physiological, psychological socio-cultural or economic conditions. “Mr. M’s elbow pain is not being effectively controlled”. “My patient is experiencing or orthostatic hypotension with mild to light headaches each morning”. • Drug therapy involved • Relationship between undesirable event & drug o Consequence of drug therapy (caused?) DTP ≠ Medication Error • Medication Errors: the 5 rights o Correct drug, dose, route, frequency & duration o Focuses n prescribing & dispensing accuracy 1) Drug Unnecessary o No valid indication o Multiple drugs when single drug would work o Non-drug therapy would be better (lifestyle) o Treating an adverse effect of another drug (including OTC’s or Herbals) o Drug, tobacco or alcohol is using the problem 2) Drug Needs to be Added Safer drug product available o Drug interaction (not dose related) o Medical condition requiring treatment o Drug regimen changed too quickly o Preventive drug therapy due to risk of disease o Allergic reaction o Contraindicated (e.g., pregnancy) o ▪ Aspirin for heart attack &/or stroke ▪ Calcium for osteoporosis 6) Dose Too High Medical condition requires addition for synergy, additive or protective effects 3) Drug Not Effective o Not the most effective drug o Medical condition is refractory ▪ o Treating high triglycerides without controlling blood glucoses first o Dose too high o Dosing frequency too short o Duration of therapy too long o Drug interactions resulting in toxic levels o Dose given or increases too quickly 7) Non-Compliance o Patient Wrong dosage form ▪ o o Immediate release morphine for chronic pain Drug not effective ▪ Antibiotic resistance 4) Dose is Too Low o Dose too low o Interval too infrequent ▪ o human ▪ Prefers not to (Why?) ▪ Forgets ▪ Can’t afford ▪ Cannot swallow administer ▪ Can’t obtain/find product or self- Rifampin and contraceptives Duration of therapy too short oral • Condition • Drug Therapy • Association between condition & drug therapy Examples • 5) Adverse Drug Reaction o Doesn’t understand instructions Stating DTP regular Drug interaction is reducing blood levels ▪ o One daily insulin?! ▪ Causes ADR (not dose related) 61 year old male experiencing gastrointestinal bleeding caused by aspirin therapy. o • 29 year old patient having breakthrough seizures due to subtherapeutic phenytoin levels. o • Vs same patient taking low dose aspirin for prophylaxis to prevent a second MI, has a history of GI bleeding due to a peptic ulcer. Vs same patient who is non-compliant due to forgetting doses 43 year old female being treated with ceftriaxone and gentamicin for a UTI has poor renal function o Vs sane patient who’s renal function gets worse while on gentamicin Prioritizing • • What needs to be fixed immediately? o Contraindications or potential harm o Problems addressing a chief complaint What problems can be fixed directly o • By you/the practitioner workng with patient & family What problems will require consultation/help PREVENTING MEDICATION -RELATED PROBLEMS (pdf file attached here) PREVENTING MEDICATION-RELATED PROBLEMS WEBINAR Dec 5, 2007 Featuring Patricia W. Slattum, PharmD, PhD, andProblems moderated by Ayn Welleford, PhD Preventing Medication-Related Virginia Alzheimer’s Commission AlzPossible Initiative INTRODUCTION Medications are probably the single most important health care technology in preventing illness and disability in the older population. Avorn J., Health Affairs, Spring 1996 Preventing Medication-Related Problems Virginia Alzheimer’s Commission AlzPossible Initiative "Any symptom in an elderly patient should be considered a drug side effect until proven otherwise." J Gurwitz, M Monane, S Monane, J Avorn Brown University Long-term Care Quality Letter 1995 Preventing Medication-Related Problems Virginia Alzheimer’s Commission AlzPossible Initiative WHAT IS A MEDICATION-RELATED PROBLEM? Medication-Related Problem (MRP) An undesirable event experienced by a patient that involves or is suspected to involve drug therapy and actually or potentially interferes with a desired patient outcome. Preventing Medication-Related Problems Virginia Alzheimer’s Commission AlzPossible Initiative “SYMPTOMS” of MRPs CHANGES IN SPEECH FALLS CONFUSION LOSS OF APPETITE DEPRESSION “SYMPTOMS” OF MRPs WEAKNESS OR LETHARGY INCONTINENCE DELIRIUM INSOMNIA PARKINSON’S-LIKE SYMPTOMS Preventing Medication-Related Problems Virginia Alzheimer’s Commission AlzPossible Initiative TYPES OF MRPs Medical condition requires new or additional drug therapy that has not been prescribed. Patient taking unnecessary drug given present condition. Correct drug, dose too low. TYPES OF MRPs Wrong drug for patient’s medical condition or age. Correct drug, dose too high. Adverse drug reaction or drug interaction. Patient not taking drug correctly. Preventing Medication-Related Problems Virginia Alzheimer’s Commission AlzPossible Initiative REASONS OLDER ADULTS ARE AT GREATER RISK FOR MRPs Multiple chronic diseases Multiple medications Multiple prescribers Physiologic changes associated with aging Under-representation in clinical trials, particularly those over age 75 Shortage of professionals with specific training to work with older adults Preventing Medication-Related Problems Virginia Alzheimer’s Commission AlzPossible Initiative REASONS MRPs ARE NOT ADDRESSED The patient has been taking this medication for many years without a problem. One provider did not prescribe all of the medications the patient is taking. Patients and prescribers are concerned that the risk of discontinuing the medication is greater than the benefit. Patients often resist changes in their drug therapy (a stereotype). The problems the patient is experiencing are not usually seen with this medication. Preventing Medication-Related Problems Virginia Alzheimer’s Commission AlzPossible Initiative PREVENTING MRP 1 Communicate with health care providers about medications Preventing Medication-Related Problems 2 Designate a medication manager 3 Keep a medication list Virginia Alzheimer’s Commission AlzPossible Initiative PREVENTING MRP 4 5 Consult with a doctor or pharmacist before taking over-thecounter medication or herbal supplements Use common sense when using medications 6 Obtain refills in a timely manner . Preventing Medication-Related Problems Virginia Alzheimer’s Commission AlzPossible Initiative TIPS FOR ADMINISTERING MEDICATIONS TO PATIENTS WITH DEMENTIA LANGUAGE ROUTINE MANAGEMENT ORGANIZATION ADMINISTRATION STORAGE Use clear and simple language. Develop a routine. Don’t assume the patient can manage medications on their own. Keep medications organized. Adapt medication administration to the patient’s needs. Store medications safely. Preventing Medication-Related Problems Virginia Alzheimer’s Commission AlzPossible Initiative PANELISTS Dr. Patricia Slattum graduated with a B.S. and Pharm.D. in Pharmacy, a Ph.D. in Pharmaceutics, and a Certificate in Aging Studies from MCV/VCU. She received further training as a geriatric pharmacy fellow at McGuire Department of Veterans Affairs Medical Center in Richmond and as an NIH-funded postdoctoral fellow in aging and drug disposition at the University of North Carolina at Chapel Hill. Dr. Slattum joined the faculty at MCV/VCU School of Pharmacy in 1996, and is currently Associate Professor and Vice Chair for Graduate Studies in the Department of Pharmacy/ Department of Pharmaceutics at VCU. Her primary responsibilities include professional and graduate teaching, clerkship training and clinical program development in assisted-living and community pharmacy practice, and geriatric clinical pharmacology research focusing on central nervous system pharmacodynamics and medicationrelated problems in the elderly. She is a member of the American Society for Clinical Pharmacology and Therapeutics, the American Society of Consultant Pharmacists, the American College of Clinical Pharmacy, the Gerontological Society of America, and the American Geriatrics Society. Ayn Welleford, PhD, is Chair, VCU Department of Gerontology, Associate Professor,VCU Department of Gerontology, and Associate Director, Virginia Geriatric Education Center. Dr. Welleford received her B.A. in Management/ Psychology from Averett College, M.S. from the Department of Gerontology and Ph.D. in Developmental Psychology from VCU. She has taught extensively in the areas of Lifespan Development, and Adult Development and Aging. As an educator, researcher, and previously as a practitioner she has worked with a broad spectrum of individuals across the caregiving continuum. As a gerontologist she currently works extensively with formal and informal caregivers to improve elder care through education. Preventing Medication-Related Problems Virginia Alzheimer’s Commission AlzPossible Initiative DISP II FINALS LECTURES PHYSICO-CHEMICAL AND THERAPEUTIC INCOMPATIBILITIES An incompatibility is defined as a change resulting and an undesirable product is formed, which may affect the safety, efficacy, appearance and stability of the pharmaceutical product. It is of three types. It includes physical, chemical and therapeutic incompatibilities. Physical incompatibility- (aka Pharmaceutical incompatibility) when two or more than two substances are combined together, a physical change takes place and an unacceptable product is formed. Interaction between two or more substances which may lead to change in color, odor, taste, viscosity and morphology. Chemical incompatibility- reaction between two or more substances which lead to change in chemical properties of pharmaceutical dosage form. As a result, a toxic or inactive product may be formed. INCOMPATIBILITIES OCCUR DURING: ▪ Compounding ▪ Formulation ▪ Manufacturing ▪ Packaging ▪ Dispensing ▪ Storage ▪ Administration of drugs ▪ The incompatibilities may be detected by changes in the physical, chemical, and therapeutic qualities of the medicine. TYPES OF INCOMPATIBILITIES ▪ Therapeutic incompatibility- It is the modification of the therapeutic effect of one drug by the prior concomitant administration of another. It may be as a result of prescribing certain drugs to a patient with the intention to produce a specific degree of pharmacological action, but have result or intensity of the action produced is different from that intended by the prescriber. The incompatibilities occur when the components of a medicine interact in such a way that properties of that medicine are adversely affected. o Physical incompatibilities o Chemical incompatibilities o Therapeutic incompatibilities PHYSICAL INCOMPATIBILITIES: https://youtu.be/OwmxFMjyKY0 ▪ When two or more than two substances are combined together, a physical change takes place and an unacceptable product is formed. ▪ Interaction between two or more substances which may lead to change in color, odor, taste, viscosity and morphology. It is also called as pharmaceutical incompatibility. ▪ Manifestations of physical incompatibility: Video Transcription: INTRODUCTION ▪ Incompatibility is defined as a change resulting and an undesirable product is formed, which may affect the safety, efficacy appearance and stability of the pharmaceutical product. DEFINITION ▪ It is defined as when two or more ingredients of a prescription are mixed together, the undesired changes that may takes place in the physical, chemical or therapeutic properties of the medicament is termed as incompatibility. o The following list outlines the various ways incompatibility between or among drug agents may be manifested. A. Insolubility: insolubility of prescribed agents in vehicle B. Immiscibility: Immiscibility of two or more liquids C. Precipitation: It occurs due to solvent is insoluble when it is added to solution. E.g.,: Mixture of prepared chalk Rx D. Liquidation: Liquefaction of solids mixed in a dry state (called eutaxia). Chalk powder – 2g Tincture catechu – 2ml Cinnamon water – 2ml A. Insolubility: insolubility of prescribed agents in vehicle The corrected prescipitation is ▪ Mixture of prepared chalk It means the inability of material to dissolve in a particular solvent system. The majority of incompatibilities is due to insolubility of the inorganic as well as organic compounds in particular solvents. Rx Chalk powder – 2g Tragacanth – 0.4g Cinnamon water up to 30ml ▪ The following factors affect the solubility of prescribed agent in vehicle and may render it less soluble. o Change in PH o Milling o Surfactant o Chemical reaction o Complex formation o Co-solvent A. Insolubility: insolubility of prescribes agents in vehicle: ▪ Any change in previous factors may lead to precipitation of drugs and change in their properties. ▪ Substances like chalk, acetyl salicylic acid, succinyl sulphothiazzole, zinc oxide, and calamine are the common examples of in diffusible solids. ▪ Some tinctures containing resins or chlorophyll may provide precipitation when added to the aqueous system. ▪ Causes: Chalk powder is not soluble in water. It gets precipitated when added to aqueous medium. These precipitates are found in diffusible in nature which results in physical incompatibility. ▪ Remedy: Use of suspending agents is necessary to suspend the precipitated chalk particles. ▪ Generally 2% X/V of compound tragacanth powder is recommended as suspending agent. B. Immiscibility: Immiscibility of two or more liquids ▪ When two such ingredients are combined resulting in a nonhomogenous product, such ingredients are called immiscible to each other and the phenomenon is called immiscibility. ▪ This manifestation appears clearly in emulsions, creams, lotions, some types of ointments. ▪ Separation in two phases is noticed in this pharmaceutical dosage form. ▪ Storage must be in room temperature to prevent separation. ▪ The following immiscibility ▪ Incomplete mixing ▪ Addition of surfactant with ▪ Unsuitable concentration ▪ False time of addition ▪ Unsuitable concentration ▪ False time of addition ▪ Unsuitable for the type of emulsion ▪ Presence of microorganisms ▪ Some bacteria grow on constituents of mixture factors lead to Acacia – 2% W/V Water – up to 60ml D. Liquefaction: Liquefaction of solids mixed in a dry state (called eutaxia). ▪ When certain low melting point solids are mixed together, a liquid or soft mass know as eutectic mixture is produced. ▪ This occurs due to the lowering of the melting point of the mixture to below room temperature and liberation to liquid. ▪ The medicaments showing type of behavior are camphor menthol, phenol, thymol, chloral hydrate. ▪ Causes: This mixture is a physical incompatibility because both the ingredients in the prescription are liquefiable of mixed together. E.g. Gelatin Arabic gum ▪ Others produce enzymes which oxidize the surfactant. ▪ Temperature ▪ ▪ Oils and water are immiscible with each other which shows physical incompatibility Remedy: These substances can be dispensed by any one of the following method. ▪ Triturate together to form liquid and mixed with an absorbent (light kaolin, magnesium carbonate) to produce the following powder. ▪ The individual medicaments is powdered separately and mixed with an adsorbent and then combined together tight and filled in a suitable container. E.g. Castor oil emulsion Rx Castor oil – 15ml Water – 60ml ▪ ▪ Causes: In this prescription castor oil is immiscible with water due to high interfacial tensions, which is a sign of incompatibility. Remedy: To overcome this type of incompatibility emulsification is necessary with the help of an emulsifying agent. ▪ The corrected prescription is ▪ Castor oil emulsion Rx Castor oil – 15ml C. Precipitation: It occurs due to solvent is insoluble when it is added to solution. ▪ PRECIPITATION: Solubilized substances may precipitate from it solution if a non-solvent for the substances is added to the solution. ▪ E.g.: Resins are insoluble in water ▪ Alcoholic solution of resins + water = precipitated resins. ▪ ▪ ▪ ▪ ▪ ▪ Aqueous dispersions of hydrophilic colloids (polysaccharide mucilage + high concentration of alcohol or salts) = precipitated colloids. High concentration of electrolytes causes cracking of soap emulsion by salting out the emulsifying agents. Vehicles (one or more organic liquids) use to dissolve medicaments of low solubility; water soluble adjuvant practically inorganic salts may be precipitated in such vehicles. ▪ Occurrence: ▪ Chemical incompatibilities occur, due to the chemical properties of drugs and additive like, ▪ PH change ▪ Oxidation-reduction reactions ▪ Acid-base hydrolysis ▪ Double decomposition These reactions may be noticed by When tinctures containing resinous matter are added in water, resin agglomerates forms in diffusible precipitates. This can be prevented by slowly adding the undiluted tincture with vigorous shake. Suspension or by adding some suitable thickening agent. E.g.: Lotion of compound tincture of benzoin o Precipitation o Effervescence o Decomposition o Color change o Explosion CHEMICAL INCOMPATIBILITIES: TYPES ▪ Based on chemical interactions ▪ Tolerated incompatibility: In this type incompatibility, the chemical interactions can be changing the order mixing the solutions in dilute forms, without or by changing the order of mixing. ▪ Rx ▪ Tincture benzoin compound – 5g ▪ Glycerin – 10ml ▪ Rose water up to 100ml ▪ Causes: Tincture benzoin compound contain resins. This change in solvent system results in an unavoidable precipitate. ▪ Adjusted incompatibilities: In adjusted incompatibility change in the formulation is needed with a compound having equal therapeutic value. ▪ Remedy: Addition of tincture with rapid stirring yields a fine colloidal dispersion. So there is no need of any suspending agents. ▪ Based on nature of chemical reaction ▪ Immediate incompatibilities: If the chemical reaction takes place, immediately after combining the prescription ingredients, they are called immediate incompatibilities. Hence, they should be dispensed only after correction. ▪ Delayed incompatibility: When the chemical reaction proceeds at a very slow rate and no appreciable visible change occurs which may develop on CHEMICAL INCOMPATIBILITIES ▪ Reaction between two or more substances which lead to change in chemical properties of pharmaceutical dosage form. As a result of this a toxic or inactive or product may be formed. keeping the product for along time are called delayed incompatibility. ▪ Intentional: When the prescriber knowingly prescribes the incompatible drugs. This method is suitable for in diffusible precipitates following steps are carried out 23. ▪ Divide the vehicle into two portions. ▪ Unidirectional: When the prescriber prescribed the drugs without knowing that there is incompatibility between the prescribed drugs. Dissolve the one of the reacting substance in one portion. ▪ Place second portion of vehicle in mortar and incorporate suitable amount of compound. ▪ Tragacanth powder (2g/100ml of preparation) with constant trituration until a smooth mucilage is produced. ▪ Add and dissolve the other reacting substance to the mucilage. ▪ Add the solution of first reactant to the mucilage slowly with constant stirring. ▪ A secondary label – SHAKE THE BOTTLE BEFORE USE ▪ Based on the prescriber: ▪ ▪ ▪ Generally reaction between strong solution proceed at a faster rate and the precipitates are formed are thick and do not diffuse readily. ▪ Reaction between the dilute solutions proceeds at a slow rate and the precipitates formed are light and diffuse readily in the solution. ▪ Hence the reaction substances should be diluted as much as possible before mixing. ▪ Precipitate yielding interactions ▪ The precipitates so formed may be diffusible or indiffusible. The method A or B is followed in dispensing the prescription yielding diffusible and indiffusible precipitates respectively. ▪ The preparation should contain a thickening agent if the precipitates is non-diffusible. Method A: ▪ Method B This method is suitable for diffusible precipitates following steps are carried out. ▪ Divide the vehicle into two portions. ▪ Dissolve the reactants in separate portions and mix the two portions by slowly adding one into other with constant stirring. Should be fixed on the container whenever method A or method B is followed in dispensing the prescription. Alkaloid incompatibility: 1. Alkaloidal salts with alkaloid substances 2. Alkaloidal salts with soluble iodides 3. Alkaloidal salts with tannins 4. Alkaloid salts with salicylates 5. Alkaloid with soluble iodides and bromides Soluble salicylates incompatibility: 1. Soluble salicylates with ferric salts 2. Soluble salicylates with alkali bicarbonates 3. Soluble salicylates benzoates with acids and ▪ The aromatic spirit of ammonia contains negligible mount alcohol. ▪ Remedy: Strchnine hydrochloride gets precipitated yielding diffusible precipitate, hence follow method A. Soluble iodides incompatibility: 1. Oxidation of iodides with potassium chloride 2. Oxidation of iodides with quinine sulphate Chemical incompatibility causing evolution of carbon dioxide gas: 1. Sodium bicarbonate with soluble calcium or magnesium salts 2. Bismuthsubnitrate sodium bicarbonate and 3. Borax with sodium bicarbonate and glycerin E.g 2: Quinidine hydrochloride mixture ▪ Rx ▪ Quinine hydrochloride – 0.12ml ▪ Sodium salicylate – 4g ▪ Water – 100ml ▪ Causes: When quinine hydrochloride combined with the sodium salicylates it forms quinine salicylates which is an in diffusible precipitate. ▪ Remedy: Hence follow method B for precipitate yielding interactions. Miscellaneous incompatibilities: 1. Soluble barbiturates with ammonium bromide 2. Potassium chlorate oxidisible substances 3. Incompatibility emulsifying agent with of 4. Color stability of dyes 5. Incompatibilities liquorices liquid extract of THERAPEUTIC INCOMPATIBILITY INCOMPATIBILITY It is the modification of the therapeutic effect of one drug by the prior concomitant administration of another. It may be as a result of prescribing certain drugs to a patient with the intention to produce a specific degree of pharmacological action, but have restore or intensity of the action produced is different room that intended by the prescriber. Eg 1: strychnine hydrochloride mixture ▪ Rx ▪ Strychnine hydrochloride solution – 6ml ▪ Aromatic spirit of ammonia – 4ml ▪ Water up to – 120ml MECHANISM: ▪ It is divided into two groups. They are ▪ Pharmacokinetic: It involves the effect of a drug on another from the point of view that includes absorption, distribution, metabolism and excretion. ▪ Pharmacodynamics: These are related to the pharmacological activity of the interacing drugs ▪ E.g., Synergism, antagonism, altered cellular transport, effect on the receptor site. Causes: ▪ The quantity of strychnine hydrochloride is more than its solubility in water (1:30) ▪ Therapeutic incompatibilities occurs due to following reasons a. Error in dosage prescription, it becomes necessary to seek clarification from the prescriber. ▪ b. Wrong dose or dosage form c. Contra-indicated drugs d. Synergistic and antagonistic drugs e. Drug interactions PRESCRIBING CONTRA-INDICATED DRUGS ▪ There are certain drugs which may be contraindicated in a particular disease or a particular patient who is allergic to it. ▪ Corticosteroids are contra-indicated in the patients having peptic ulcers ERROR IN DOSAGE ▪ Many therapeutic incompatibilities result from errors in writing or interpreting the prescription order. The responsibility of the pharmacist becomes to check the prescription intensively and if he finds these types of errors he should immediately consult the prescriber for the clarification. ▪ The most serious type of the dosage error in the dispensing is overdose of a medication ▪ The penicillin and sulphur drugs are contraindicated in the patients who are allergic ▪ E.g., Atropine sulphate capsules ▪ ▪ Rx Vasoconstrictors are hypertensive patients. ▪ Atropine sulphate – 0.005g ▪ Barbiturates and morphine should not be given to the asthmatic patients. ▪ Phenobarbitone – 0.015g ▪ Aspirin – 0.300g ▪ ▪ Causes: In this prescription, the quantity of the atropine sulphate in each capsule is more than its recommended dose. Remedy: Before prescribing such substances a doctor must be careful. ▪ If he does not, a Pharmacist shows his caliber to point out such type of the doctor’s error. ▪ Such must immediately be referred back to the concerned doctor and get corrected. ▪ Remedy: The prescription is referred back to the prescriber to correct the overdose of the atropine sulphate ▪ The recommended dose of atropine for a single capsule is 0.25 to 2mg WRONG DOSE OR DOSAGE FORM ▪ There are certain drugs which have quite similar names and there is always a danger of dispensing the wrong drug. contra-indicated in PRESCRIBING SYNERGISTIC OR ANTAGONISTIC DRUGS ▪ When two drugs are prescribed together, they tend to increase the activity of each other which is known as SYNERGISM. ▪ When two drugs are prescribed together, they tens to decrease the activity of each other which is known as ANTAGONISM ▪ E.g., Prednisone and Prednisolone ▪ Digoxin and Digitoxin ▪ E.g., ▪ Some times many drugs are available in the different dosage forms and hence, if the dosage form is not clearly mentioned on the ▪ A combination of aspirin and paracetamol increases the analgesic activity. ▪ A combination of penicillin and streptomycin increases the antibacterial activity. ▪ Amphetamines show its antagonists effect with the barbiturates. ▪ Remedy: The prescription is referred back to the prescriber for necessary corrections. DRUG INTERACTIONS Drug Interactions: Drug interactions are changes in a drug’s effects due to recent or concurrent use of another drug or drugs (drugdrug interactions), ingestion of food (drug-nutrient interactions), or ingestion of dietary supplements (dietary supplement-drug interactions). More drug = More interactions DRUG INTERACTIONS ▪ The effect of one drug is altered by the prior or simultaneous administration of another drug. The drug interaction can usually be corrected by the proper adjustment of dosage if the suspected interaction is detected. ▪ E.g., Tetracycline capsule – 250mg capsules ▪ Direction: Take one capsule every 6 hours with milk. ▪ Causes: Tetracycline is inactivated by calcium present in milk ▪ So, it should not be taken with milk. ▪ Remedy: In this prescription, the therapeutic incompatibility is unintentional. ▪ So, the prescription is referred back to the prescriber to change the direction CONCLUSION: ▪ Incompatibility is defined as a change resulting and an undesirable product is formed, which may affect the safety, efficacy, appearance and stability of the pharmaceutical product. ▪ It is of three types. It includes physical, chemical and therapeutic incompatibilities. ▪ The below described article gives the detailed information about the types, causes and how to overcome these types of incompatibilities. ▪ The occurrence of chemical incompatibilities can be overcome by two methods which include method A&B. A drug-drug interaction may increase or decrease the effects of one or both drugs. Clinically significant interactions are often predictable and usually undesired (see Some Drugs With Potentially Serious Drug-Drug Interactions). Adverse effects or therapeutic failure may result. Rarely, clinicians can use predictable drug-drug interactions to produce a desired therapeutic effect. For example, coadministration of lopinavir and ritonavir to patients with HIV infection results in altered metabolism of lopinavir and increases serum lopinavir concentrations and effectiveness. Mechanism Narrow margin of safety Examples Antiarrhythmic drugs (eg, quinidine) Antineoplastic drugs (eg, methotrexate) Digoxin Lithium Theophylline Warfarin Alprazolam Amitriptyline Atorvastatin Carbamazepine Clozapine Corticosteroids Cyclosporine Diazepam HIV protease inhibitors Imipramine Extensive metabolism by Lovastatin certain hepatic enzymes Midazolam Olanzapine Phenytoin Sildenafil Simvastatin Inhibition also can occur after ingestion of grapefruit products. Tacrolimus Tadalafil Theophylline Triazolam Vardenafil Warfarin In therapeutic duplication, 2 drugs with similar properties are taken at the same time and have additive effects. For example, taking a benzodiazepine for anxiety and another benzodiazepine at bedtime for insomnia may have a cumulative effect, leading to toxicity. Aprepitant Boceprevir Cimetidine Ciprofloxacin Clarithromycin Cobicistat Conivaptan Diltiazem Erythromycin Inhibition of certain Fluconazole hepatic enzymes Fluoxetine Fluvoxamine Itraconazole Ketoconazole Paroxetine Posaconazole Ritonavir Telaprevir Telithromycin Verapamil Voriconazole Barbiturates phenobarbital) Bosentan Induction of certain Carbamazepine hepatic enzymes Efavirenz Phenytoin Rifabutin Rifampin St. John’s wort Drug interactions involve • Pharmacodynamics • Pharmacokinetics In pharmacodynamic interactions, one drug alters the sensitivity or responsiveness of tissues to another drug by having the same (agonistic) or a blocking (antagonistic) effect. These effects usually occur at the receptor level but may occur intracellularly. (eg, *Any drug to be used concurrently with one of these drugs should be thoroughly evaluated for possible interactions. Even when used alone, these drugs may have serious adverse effects. Concurrent use of another drug that increases the action of these drugs further increases risk of adverse effects. For additional research on potential drug-drug interactions, consult a reliable source. In pharmacokinetic interactions, a drug usually alters absorption, distribution, protein binding, metabolism, or excretion of another drug. Thus, the amount and persistence of available drug at receptor sites change. Pharmacokinetic interactions alter magnitude and duration, not type, of effect. They are often predicted based on knowledge of the individual drugs or detected by monitoring drug concentrations or clinical signs. Minimizing drug interactions Clinicians should know all of their patients’ current drugs, including drugs prescribed by other clinicians and all over-the-counter drugs, herbal products, and nutritional supplements. Asking patients relevant questions about diet and alcohol consumption is recommended. The fewest drugs in the lowest doses for the shortest possible time should be prescribed. The effects, desired and undesired, of all drugs taken should be determined because these effects usually include the spectrum of drug interactions. If possible, drugs with a wide safety margin should be used so that any unforeseen interactions do not cause toxicity. Patients should be observed and monitored for adverse effects, particularly after a change in treatment; some interactions (eg, effects that are influenced by enzyme induction) may take ≥ 1 week to appear. Drug interactions should be considered as a possible cause of any unexpected problems. When unexpected clinical responses occur, prescribers should determine serum concentrations of selected drugs being taken, consult the literature or an expert in drug interactions, and adjust the dosage until the desired effect is produced. If dosage adjustment is ineffective, the drug should be replaced by one that does not interact with other drugs being taken. Which aspect of drug administration is most likely to control the effect of a drug on a patient? - Drug concentration at the site of action Which of the following best defines the therapeutic index of a drug? - Ratio of the minimum toxic concentration to the median effective concentration Which of the following is most likely to result from increasing the dose of a drug with a small therapeutic index? - Increased probability ineffectiveness of toxicity or https://youtu.be/aDsW8tx1KsY Video transcription: Which of these three people is doing something risky is it the one who takes their cholesterol medication with grapefruit juice the one who takes acetaminophen pain relievers for a sore ankle before going out for drinks or the one who's on a blood thinning medication and takes a nap print for a headache actually all of them are has inadvertently created a drug interaction that could in extreme cases lead to kidney failure liver damage or internal bleeding drug interactions happen when a combination of a drug with another substance causes different effects than either would individually foods herbal legal drugs and illicit substances can all cause drug interactions most drug interactions fall into two categories some take place when two substances affect influence each other directly in other cases one substance effects how the body processes another like how it is absorbed metabolised or transported around the body blood thinners and aspirin for example have similar effects that become dangerous when combined both prevent blood clots from forming blood thinners by preventing the formation of the clotting factors that hold clots together and aspirin by preventing blood cells from clumping into groups that become clots individually these effects are usually safe but taken together they can prevent blood clotting to a dangerous extent possibly causing internal bleeding while blood thinners and aspirin are generally harmless when taken individually interactions where one substance exacerbates the effects of another can also take place between drugs that are independently harmful cocaine and heroin are each dangerous and those dangerous compound when the two drugs are combined even though their behavioral effects may feel like they cancel each other out cocaine is a stimulant and many of its attacks like increased heart rate caused the body to need more oxygen but heroin a depressant slows breathing reducing the body's oxygen supply just needs more this combination strains the organs and can cause respiratory failure and death the interaction between grapefruit juice and certain medications and a class of cholesterol lowering drugs called statins has to do with drug metabolism the liver produces enzymes molecules that facilitate the breakdown of substances that enter the body enzymes can both activate drugs by breaking them down into their therapeutic ingredients from more complex molecules and deactivate them by breaking harmful compounds down into harmless metabolites there are many many different enzymes each of which has a binding site that fits a specific molecule grapefruit binds to the same enzyme as statins making less of that enzyme available to breakdown stats so combining the two means that a greater concentration of the drug stays in the bloodstream for a longer period of time potentially causing kidney failure alcohol can also alter the function of the enzyme that breaks down acetaminophen the active ingredient in pain relievers like Tylenol and paracetamol when someone takes acetaminophen some of it is converted into a toxic substance at the recommended dose there isn't usually enough of this toxic byproduct to cause harm but heavy drinking can alter enzyme activity so more of that byproduct is produced potentially causing liver damage even with what's usually a safe dose of acetaminophen meanwhile the herbal remedies St. Johns wart increases the liver's production of a particular enzyme that means the drugs this enzyme is responsible for breaking down get metabolised faster before they can have their therapeutic effects in spite of the dizzying number of possible interactions most of the dangerous interactions with commonly used drugs are well known and new developments in science are helping us keep better track of drug interactions than ever developing AI programs that can predict the side effects of drug interactions before they occur using information about the landscape of protein interactions within your body for the new drugs that are being developed all the time super computers are being used to find potential interactions while those drugs they're still in development when you take medicine how does it know where it needs to go following the path of medicine as it travels through the body in this video. What are the Cytochrome P-450 enzymes? https://youtu.be/rUSOtkvZx_k Video transcription: You'll notice that the effects of the drug wears off in a few hours have you ever wondered how does this happen in your body has this question kept you awake at night I'm sure it does because I have for you today this happens because of therapy for 15 years enzymes involved in drug metabolism and bioactivation is the breakdown of xenobiotics and human specially substances that you introduce into your body therefore ensure body such as medicine or drugs that you intake the location of this breakdown is predominantly the liver the biggest role of cytochrome P450 enzyme is to turn drugs into soluble molecules so that it can be excreted out properly active set of cytochrome P450 contains a heme Arts Center specifically hamby it is also present in hemoglobin and myoglobin keep consist of this complex organic rank structure called purpose out of his mouth down to the fourth night of the porphyrin ring system forming a plea he must also collecting to another molecule the system so the irony is ultimately held in the center with a nitrogen of the ring as well as the style of assisted residue holding it from the bottom within the circle P for 50 different kinds of P 4:15 at science but they all the chance in the structure this has 13A helices and five Peter shoots presented as purple coils and Pi helices as green coils strands as Red Arrows and Peter loops are represented as yellow tubes it has also been labeled present just prior to the L Helix architecture is required to protect the system and hold it in place in order to accept protons involved in the activation is the portion of Helix near the heel area action between the oxygen and iron is taking place oxygen interests on the opposite side of style and it always interested in goal it never enters into matter so let's discuss how exactly to decide poopy for 15 times work at the beginning the art in the Center for 50 will be in the Ferris state three plus state the army is and represents the truck very permission the first step involves coordinating or combining with the truck and giving the enzyme side of trophy for 50 with the substrate are each complex in the second step complex will get used by NADPH will get off sized to energy while the electron gets transferred to the enzyme substrate complex state of the enzyme is FE2 plus involves reduction of the enzyme complex and oxidation of the NADPH in the first step oxygen molecule concept and electron from iron gets transferred over to the oxygen and it then produces this conflict heart oxidized it has lost an electron whereas oxygen has been reduced it has schemed an electron step forward similar to Step 2 and if he gets oxidized to an easy plus schatzman selected on to the conflict however note that electron is not accepted by the iron atom instead it is accepted by the oxygen atom hotels are added auction complex two electrons as one of the oxygens from the complex gets transferred to the protons which differ producing water molecule the fact that two oxygens in the complex are currently bonded in order to break the bond water molecule needs to be formed steps 125 for the next step with the truck actually gets modified in this chapter hitting oxygen with it transferred over to the substrate is both and kinetically favorable for this to occur what's this transfer occurs in time is able to release the metabolite the product ROH we left with a rational for 15 inside and its default state the cycle all over again ask her to sleep into two parts metabolism production or hydrolysis with the most common biochemical process that occurs oxidation oxidation is kind of nice for 15 inside as we just saw the mechanism cycle of statically for 15 enzymes reactions convert appearance drug to a more polar water soluble metabolite by unmasking earth stirring the polar functional group however after phase one reactions the resulting from metabolite is often still chemically active before it can be created conjugation reactions which is attached to the drug muscle groups increases the water solubility of the metabolite as well as decreases the pharmacological activity she was happy and active treated phase one metabolism and it's converted to salicylic acid and phase two is conjugated with either glycine or clicker on IC acid forming a range of ionized metabolites that can then be excreted in the urine. Hepatic enzymes: Inducers and Inhibitors https://youtu.be/nWjoNa8nXr4 Video transcription: Sickfaces.com and BS crap GPS and you monex to help you quickly remember the inducers and inhibitors of the cytochrome P450 enzymes these are a group of enzymes present in the liver that play a role in metabolizing a range of different compounds but the activity of these enzymes can be affected by certain compounds known as either inducers or inhibitors this means that certain compounds can cause an increase or decrease in the effect of other medications the patient may be taking will do inhibitors first meaning they decrease the activity of cytochrome P450 enzymes and so any drug that is metabolised by these enzymes will now be cleared slower and therefore may have a greater impact than expected sickfaces.com group is the traditional numonic but we're making a small change and adding a T to make it stick faces.com group which will make it easy to remember that these are inhibitors as you can see by the word stop spelled in yellow so S stands for sodium valproate which is an antiepileptic drug and AT we just added is particularly P which is an antiplatelet medication I for isoniazid see are the first scene is cimetidine and anti H2 receptor drug that inhibits stomach acid production Kate is key to console and F is fluconazole both of which are antifungal drugs here is alcohol use specifically acute consumption or binge drinking a is also the antiarrhythmic drug amiodarone II C reminds us of ciprofloxacin a fluoroquinolone as well as chloramphenicol he is for every throw myson and as is for the cell phone emits the third C is to remind you of cranberry juice oh it's for omeprazole which is a proton pump inhibitor that is used to reduce stomach acid production M is metronidazole an antibiotic that is commonly used in GI infection and it has antiparasitic properties finally we have group to remind you that grapefruit juice is also inhibitor of the cytochrome P450 enzymes This is why you shouldn't see cranberry and grapefruit juice being given for breakfast at the hospitals OK so let's do the inducers now these increase the activity of the cytochrome P-450 enzymes and so the drugs that the patient is taking that are metabolised by cytochrome P-450 enzymes will now get cleared faster and therefore be less effective than we expected BS crap GPS is the new monik so to help you remember that these induce cytochrome people 50 enzymes think about how having a crap GPS would induce anger or rage or whatever so he stands for the barbiturates and the first S is for Saint Johns wort C is for carbamazepine which is an anti epilepsy drug and R is for rifampin also known as rifampicin an antibiotic that is used against things like tuberculosis a is once again for alcohol but this time it's more chronic use of alcohol rather than binge drinking or cute consumption P is for phenytoin yet another anti seizure drug and next we have G for grease or full vin which is an antifungal drug P is for phenobarbital our final anti seizure medication that also works as a sedative finally we have S which stands for sulfonylureas these are class of drugs used to increase insulin production in diabetics How to remember drug interactions: https://youtu.be/AdnLUxdMvHY Video transcription: Hi and welcome back to memorable psychopharmacology I was at a psychopharmacology conference recently and the number one request I received was to take the memorable psychopharmacology approach and apply it to drug drug interactions so without further ado let's dive into the often confusing world of psychotropic drug drug interactions at its core a drug drug interaction is what happens when one drug has an effect on another drug often in a negative or unintended way this generally happens by one of two mechanisms either the two drugs have an effect that is too strong in combination whether that's a beneficial effect or a side effect or one drug changes the metabolism have another drug the first type of interaction is seen in something like serotonin syndrome when a patient is given two different medications that both increase serotonin the body receives too much serotonin ergic stimulation and serotonin syndrome can occur the second type of interaction involves changes in drug metabolism when a drug enters their bodies it diffuses through the bloodstream and exerts its intended effects on the target organ which in the case of psychopharmacology is generally the brain for the purposes of this lecture let's say that this drug is sertralin and necessary which is commonly used to treat depression the drug continues to circulate through the bloodstream and exert its effect until it is metabolised by the liver at which point it is rendered inactive and excreted from the body some medications are excreted by the kidneys but this is rare in psychopharmacology as we'll find out later drug metabolism in the liver is the most common way that one drug can interact with another for example let's say that this patient taking searcher lean also begins taking a mood stabilizer in this case carbamazepine carbamazepine is a known inducer because it speeds up the metabolism of certain drugs so for this patient the metabolism sertralin will increase leading to an overall net decrease in the amount of search ralin in their system in contrast let's say that this patient taking searcher lean instead also begins taking a medication for HIV such as rotana veer in contrast to carbamazepine ritonavir is an inhibitor of hepatic enzyme systems and slows down the metabolism of certain drugs so for this patient the metabolism is searchline will decrease leading to an overall net increase in the amount of searchline in their system this concept is on the surface very simple and inducer will speed up clearance of a drug from the bloodstream leading to an overall net decrease in drug levels while an inhibitor will slow down clearance of a drug resulting in an overall net increase in drug levels where it gets complicated is the fact that there's not just a single enzyme system in the liver that is responsible for metabolising drugs rather there are dozens each going by seemingly arbitrary and difficult to remember sub names like 2D63A four 1822B6 and so on each individual drug is metabolised by each of these systems to varying degrees for example sertraline is metabolised by the three A4 enzyme system whereas another Sir I like fluoxetine is not if you gave someone a 3A4 inhibitor the levels of searcher lean in the body would go up while the levels of floxy teen would not be affected in contrast floxin is metabolised by the 2D6 enzyme system so if you instead give someone a 2D6 inhibitor the levels of locks it in in the body would go up while the levels of sertraline would not be affected a further complication is the fact that certain drugs known as pro drugs are actually activated by liver metabolism rather than being inactivated for example the pain medication codeine is converted into its active form morphine in the liver by the 2D6 system coding itself is not a very powerful analgesic but morphine is in the presence of a powerful 2D6 inhibitors such as lock cyteen however this transformation into morphine is blocked rendering coding a very ineffective analgesic to complicate things even further the effect that certain drugs have on each enzyme system is not a binary yes or no on or off type of system rather there is a range of effects that a drug can have for example a drug LifeLock cyteen is a strong 2D6 inhibitor while search ralin is only a moderate 2T6 inhibitor and another SSRI italic ram is a weak 2D6 inhibitor finally to add that last wrinkle of complication many enzyme systems in their liver are affected not only by other drugs but by genetics as well for example the inherent activity of the two D6 enzyme responsible for metabolising floxy teen is genetically determined this means that some people are slow metabolizers and operate as if they have a built in 2D6 inhibitor leading to increased levels of medications like block city and even at the same dose as somebody else other people are inherently rapid metabolizers leading to lower levels of loxitane at the same dose putting this all together the amount of complexity can become almost overwhelming the different effects inducers and inhibitors can have the multiple enzyme systems the distinction between drugs and prodrugs this strength of interaction and the contribution from genetics combined can make for a seemingly infinite amount of information to know because of this complexity computerized alert systems have been developed that will let you know when you're about to prescribe 2 drugs that can interact with one another while helpful in some ways given the vast number of possible interactions these systems are prone to alert fatigue where the user is warned of so many possible interactions that they just start to ignore all of them so how can we best address this we can't completely ignore drug drug interactions because they have the potential to seriously impact our patients however trying to memorize every single interaction is also simply not feasible to help make sense of this I recommend an approach where you focus on clinically significant interactions while this can be a difficult distinction to make between those that are clinically significant those that aren't I personally believe that a clinically significant interaction is one where any of the following is true the interaction is particularly strong the outcomes of ignoring the interaction are bad and or the effect is not subjectively noticeable we'll go over each of these concepts 1 by 1 as mentioned previously different drugs can have different effects on enzyme systems for example a strong inhibitor like fluoxetine can cause a five times or greater increase in the concentration of certain drugs while a weaker inhibitor like satala pram will not cause any more than a doubling of the concentration because of this we'll focus our discussion on those interactions that are particularly strong within the field of psychopharmacology there are a few particularly strong inducers that you should be aware of both carbamazepine and phenobarbital are very strong inducers of the three A4 system and you will see them frequently mentioned during this lecture you can remember these by thinking of them as carb and Barb conversely there are a few very strong inhibitors that you should know about specifically bupropion fluoxetine and paroxetine are each very strong inhibitors of the two D6 system which you can remember by thinking that bupropion fluoxetine and peroxy teen can cause big freaking problems and drug metabolism as we'll see soon second we'll focus on the interactions where the outcome is particularly bad if it's ignored a change in someone's medication for seasonal allergies for example can lead to increased allergic symptoms but is unlikely to result in significant morbidity or death in contrast a change in someone's chemotherapy regimen can result in increased chances of cancer recurrence finally we will pay particular attention to those interactions with the effect of our changes are not immediately or subjectively noticeable changing the metabolism of someone's benzodiazepine's for example is often noticeable and the patient will complain of over sedation or conversely of the medication not having any effect at all in contrast changing the metabolism of someone's blood pressure medications is not likely to be noticeable by the patient until things progressed to the point where a bad outcome like a stroke occurs to remember all the clinically significant interactions that you will run into in the world of psychopharmacology use the new monik I can have fun heartily outsmarting warring drugs will go over each of these one by one I is for immune and applies to things like antibiotics and immunosuppresants many antibiotics such as clarithromycin can also be strong inhibitors of certain enzyme systems in addition maintaining appropriate levels of immunosuppresants such as cyclosporine is crucially important for patients with transplanted organs as too little can cause graft rejection while too much can cause toxicity many of these medications are metabolised by the 384 system so carbamazepine and phenobarbital more carbon Barb can induce 3A4 and cause drastic reductions in immunosuppresants levels Ken is for cancer and in particular breast cancer specifically the medication tamoxifen given for certain types of breast cancer is actually a prodrug that must be metabolised by the 2D6 system into its pharmacologically active form if you are giving your patient a strong two D6 inhibitor such as bupropion fluoxetine or paroxetine you can prevent the conversion of tamoxifen into its active form thereby increasing the risk that your patient will experience a recurrence of cancer this is as the mnemonic will remind you of big freaking problem in addition many other chemotherapy agents will interact with the enzyme systems and potentially negative ways so be extra cautious for any patient being actively treated for cancer have is for HIV many of the medications used to combat HIV like the protease inhibitors are strong 3A4 inhibitors therefore there's a chance that they will interact with the patients prescribed psychotropics in particular benzodiazepine's and many antipsychotics will have an increased plasma level for patients taking a protease inhibitor so you need to be careful to lower their doses to avoid oversedation and extrapyramidal side effects fun is for fungal medications many antifungal agents such as fluconazole are strong 3A4 inhibitors and like the protease inhibitors can increase the effect of certain benzos and antipsychotics Hartley is for heart conditions several classes of medications that are used to treat heart conditions including arrythmias such as lidocaine and certain beta blockers such as carvedilol and metoprolol are metabolised by the 2D6 system so their levels can raise drastically in the presence of a strong two D6 inhibitors such as propane flexity nor peroxy teen sudden changes in these medications can lead to a rythme as hypertension and cardiogenic shock so like with tamoxifen this can be a big freaking problem out is for oral contraceptives specifically you must be mindful to avoid certain medications for patients taking oral contraceptives to avoid adducing their metabolism and rendering the contraception ineffective for example patients prescribed carbs or Barb should not be taken in oral contraceptive and will need to seek alternative methods of contraception smarting is for seizures many anti epileptics including but not limited to those used as mood stabilizers for bipolar disorder have significant drug drug interactions for patients taking an anti epileptic you need to be careful not to give them an inducer which could lower the seizure threshold and possibly provoke another seizure one drug drug interaction involving antiepileptics that deserves special mention is between valproate and lamotrigine these medications are both used as mood stabilizers but given that valproate works for mania and lamotrigine works for depression it is not at all uncommon to see these being combined valproate is not the strongest inhibitor but has enough of an effect that when it is combined with lamotrigine the plasma levels of lamotrigine are often significantly higher given that the major side effect of lamotrigine Stevens Johnson syndrome is directly related to the dose and frequency of lamotrigine this actually becomes crucially important to avoid an unnecessary increase in the risk of Stevens Johnson syndrome make sure to use half the dose of lamotrigine you normally would and consider titrating the dose half as fast you can remember this by thinking of Valhalla the majestic hall located in the afterlife of Norse mythology by thinking of Valhalla you can remember that when you use valproate you should use half the dose of lamotrigine to avoid killing your patient and sending them to Valhalla next warring is for warfarin and other anticoagulants warfarin is metabolised by various enzyme systems in the liver given the narrow therapeutic window for warfarin being effective rather than toxic this can result in clinically significant interactions between inducers like carbon Barb which can lead to a subtherapeutic INR inhibitors like valproate and disulfiram which can increase INR and cause pathological bleeding warfarin is not the only anticoagulant subject to drug drug interactions as other anticoagulants such as rivaroxaban can be affected by carbon Barb as well finally there's a theoretical risk that SSRI's and other blockers of serotonin reuptake could interfere with platelet function as platelets cannot synthesize serotonin and instead rely on pre uptake to use it for clotting however this risk appears to be more theoretical than actual and most of the evidence for any interaction exists is anecdotal finally drugs is for diabetes many of the medications used for diabetes and particularly the secretagogues like clinic Lipton are metabolised by the 384 system so our old friends carbon Barb can return to mess things up here as well on a practical level you can use the I can have fun heartily outsmarting warring drugs mnemonic as a quick screen when reviewing your patients problem list and medication regimen if they have any of these conditions or are taking medications related to them be extra mindful to look for drug interactions from any medication you start so you've done your due diligence for your patient by making sure that they're not on any of these high risk medications however you still have the sense that you're missing something that's because you might be besides the medications we prescribe there are many substances that our patients take without our knowledge or consent that can wreak havoc with drug metabolism as well so when you have a nagging worry you've forgotten something despite having checked medication list consider counseling your patients on the following substances N is for nicotine tobacco use results in the induction of the one A2 system which is responsible for metabolising many of the drugs we use commonly in psychiatry including antidepressants and antipsychotics given that up to 70% of people with schizophrenia in the United states smoke frequently this is something to be aware of as sudden changes in smoking habits such as quitting smoking can cause levels of antipsychotics to fluctuate wildly interestingly it's not the nicotine per say that induces the enzymes but the aromatic hydrocarbons and tobacco smoke so a patient switching from cigarettes to nicotine replacement may experience a major shift in the levels of their psychotropic medications a is for alcohol alcohol acts as an inhibitor of several enzyme systems in the liver when used on an acute basis however chronic alcohol use actually causes liver damage which acts as an inducer of enzyme systems G is for grapefruit juice grapefruit juice is a commonly available 3A4 inhibitor like carbon Barb however just because it can be picked up off of any grocery store counter does not mean that it's benign grapefruit juice related drug interactions include many of the medications that we use in psychiatry including antidepressants antipsychotics benzodiazepine's stimulants and mood stabilizers were is for Saint Johns wort St. Johns wort is a dietary supplement which is available without a prescription for use as an antidepressant however it is also a strong inducer of 384 and can interact with all of the conditions mentioned in that I can have fun hardly outsmarting warning drugs mnemonic so remember to use the mnemonic nagging worry when you're concerned about non prescribed drug drug interactions to wrap up we're going to go over a few last high yield tips to help you tie psychotropic drug drug interactions together first as mentioned earlier nearly all the interactions we have discussed so far have to do with enzyme systems in the liver this is because virtually all the medications used in psychiatry are hepatically metabolised the only real exceptions to this are gabapentin acamprosate and lithium all of which are renally metabolised you can remember this by thinking that the kidneys filter gal drugs just like they do a gallon of water in particular with lithium you need to make sure to avoid other medications that could damage the kidneys and lead to lithium toxicity though this topic was covered in more detail in the section on lithium another point of review is that benzodiazepine's like most psychotropics are metabolised by the liver so they should be avoided for patients with the paddick dysfunction however 3 benzodiazepine's are safe to give even with a diseased liver and these are oxazepam temazepam and law Raza Pam a classic mnemonic for this is to remember that they are metabolised outside the liver however this mnemonic isn't exactly accurate as oxazepam temazepam and law Raza Pam are all metabolised in the liver the difference is that they are metabolised by conjugation meaning that they don't have active metabolites this means that the half lives are the same as always even in advanced liver disease so if you're gonna use the outside delivery mnemonic and it is very handy please also remember that on a scientific level it's not 100% accurate finally these drug drug interactions involving psychotropics have been covered in more depth in their respective lectures make sure to review them at the rusty serotonin syndrome is reviewed in the lecture on neurotransmitters hypertensive crisis is covered under antidepressants and the QTC prolonging medications like sital apram and separate zone are in their respective sections and now a final take home point while we've covered some of the major drug drug interactions to be aware of there's simply no way to cover them all in addition your patients will have genetic differences in enzyme systems that will further complicate things even if they're not taking any other drugs in psychopharmacology it's important to be mindful of these variations and to always try to find the minimum effective dose because we are titrating to an effect rather than a specific dose or plasma level for most of our medications it's wise to always started at a lower dose range and increase only when it's clear that the current dose is not doing the job so remember to start low and go slow A quick overview of what we've learned with in psychopharmacology carbamazepine and phenobarbital or carbon Barb are the major inducers while bupropion fluoxetine and peroxy teen are the major inhibitors causing big freaking problems you can remember the medical conditions and medication class is most often responsible for significant drug drug interactions with psychotropics using lemonick I can have fun hardly outsmarting warring drugs with the additional nagging worry mnemonic to recall non prescription drug interactions nearly all psychotropics are hepatically metabolised except for the gal drugs gabapentin acamprosate in lithium which are renally excreted just like a gallon of water finally the OTL benzos oxazepam temazepam and law Raza Pam are practically excreted outside the liver and are safe to use in patients with hepatic failure. 5 tips to avoid drug interactions: Video transcription: https://youtu.be/hzXLHHsa5bw Taking multiple medicines have you heard of medicine interactions because it could affect how well they work I'm gonna teach you five simple tips to help avoid them so you see these books and these and this and this and all these I think we got it supposes to do these types of books at university so we can fully understand why and how medicines interact now I'm not going to do is give you specific interactions and explain why they happen because will be here forever but what I will do is give you a basic knowledge but how medicines interact and then we move on to tips So what we say medicines interaction it doesn't mean that it combine them in body cause a chemical reaction of some sort in fact here's a simplified version of what medicines interaction means a drug supplemental food can affect the absorption of a drug in our body its distribution breakdown and excretion so because of this interaction the level of Douglas now body can either increase or decrease and I hope that made sense so medication interactions can be very dangerous let's say for example you take a medicine that helps to thin the blood and you take another medicine or a herbal remedy or a supplement or a food and interacts with it and it suddenly increases its level so you certainly are a higher risk of bleeding or introduces this level and you suddenly at a higher risk of having a clot now it's unrealistic for patients to memorize every single interaction with their medicines but following these simple tips can go along way tip one know exactly why you're taking each medicine let's face it drug names can be very difficult to pronounce and if your listing your medicines to a health care professional and let's say you say incorrectly then the potential interaction could go unnoticed so I thought I'd give you three of my favorite mispronunciations first I've got lanzerotti instead of lansoprazole and body pain instead of amlodipine and ramipril which I call it the Italian version of saying ramipril which actually got me thinking if there's any health care professionals watching leave a comment below with your favorite mispronunciation because we could potentially do a future video on this now back to tip one so if you say the name of the medicine followed by why you actually take it the health care professional is much more likely to know which medication you're on and be able to check for interactions with current medication that you're taking now if you're gonna find it difficult to remember or you taking a few different medicines you can always ask your pharmacy to add the reason why you're taking it on the pharmacy label that they stick on or instead you can carry a repeat medication list with you but make sure it's up to date tip two know how to take your medication so should it be taken with food on an empty stomach or should you avoid any certain types of food like dairy for example if you're not taking it right it can reduce his absorption its effectiveness or even cause irritation of the stomach lining now this information can be found in the patient information leaflet which comes with every medicine the pharmacy dispensing label or just ask your pharmacist that tell you everything you need to know I've also left a link in the description below where you can search each medicine find out more information three layer pharmacy know about all the medicines that you take pharmacies don't have access to your medical records so if you've been started on a new medication it may not know about it so when you Frances handy medicines to let them know about it and it might be able to give you some really useful tips so you can get the most out of your medicine which brings us on nicely to tip for supplements herbal remedies over the counter medicines these can all again interact with new medication so let your healthcare professional know so they can check for interactions chip five speak to your amazing pharmacist I always ask patients to bring in all their medicines so either a list or actually bring them into the pharmacy so they're prescribed medicines not prescribed medicines herbal remedy supplements bringing everything and then we can sit down and have a look at them and identify any interactions between them and give you the best advice so you can get the most out of your medicine and by the way this is something that all pharmacists will happily do for their patients so utilize their expertise and get the most out of your medication medication interactions are a very serious issue and hopefully these simple precautions will help to reduce them and if you know anyone who takes medication will probably find this information useful then share what you've learned with them today or if that takes too long to share the video with them. HOW TO USE HANDBOOK OF INJECTABLE DRUGS/INCOMPATIBILITY https://youtu.be/W040tqzhPjw Video transcription: With lightning fast search results and continually updated information ashp's interactive handbook on injectable drugs continues to set the standard for Ivy compatibility get started by typing a drug into the search box you will notice the autocomplete function is there to guide you start your search with either the generic or brand name of the injectable medications after you perform a search results quickly appear inside the search result box on the left as you click on drugs from your search results they appear on my drug list box you may also browse drugs by their starting alphabet as you can see compatibility results are instantly generated as you build your drug list once you're satisfied with your results you can also easily print them if using the online application the results may also be filtered based upon compatibility results and type there is a multiple drug search function allowing you to simultaneously check and unlimited number of medications for two drug combination compatibility to view a drugs monograph click on the icon next to the drug name the monograph will open in a new browser or tab depending on your setting tabular compatibility information is available with links to the references for the test results clicking on a reference will send you to a list of references and from there you can access the pub Med abstract to start over just click the clear button there's no limit to the number of medications for testing the updated interactive handbook now features appended medwatch safety and wall chart custom views of compatibility results also with his release suitable for home and other infusion practices is the addition of concisely formatted beyond new stating recommendations for parenteral drugs and nutritional solutions from extended stability for parenteral drugs by Corinne Bing and on an Oval skiba studios as a subscriber be sure to look out for regular electronic updates throughout the year did you know your subscription to the interactive handbook also provide you with access to the app for iOS or Android devices download the app and you'll have access to Ivy compatibility instability information wherever you go For more information visit www.ashp.org/H I D online packages are available with print version as well Which among the following methods apply to products forming indiffusible precipitates? - METHOD B ***/ 5. Incompatibilities occur during the following processes EXCEPT: - Patient counselling / 6. The following are factors leading to immiscibility EXCEPT? - Thorough mixing / 7. Which of the following fail to describe chemical incompatibility? - Modification of the therapeutic effect of one drug by the prior concomitant administration of another drug / 8. Which of the following fails to describe physical incompatibility? - None of the statements fail to describe physical incompatibility. / QUIZ ANSWERS: 1. A physical incompatibility described by the inability of a solid solute to dissolve in a particular solvent system. INSOLUBILITY / 2. A physical incompatibility that involves precipitation of solubilized substances from its solution whenever a non-solvent substance is added to the solution. An example is the addition of water to alcoholic solutions of resins leading to the formation of precipitated resins PRECIPITATION / 3. A subtype of chemical incompatibility (based on nature of chemical reaction) that takes place immediately after combining the prescription ingredients and should only be dispensed after correction. - Immediate incompatibility / 4. In an incompatibility involving precipitation, such precipitates formed may be diffusible or indiffusible. 9. Which of the following holds true for therapeutic incompatibility? - Modification of the therapeutic effect of one drug by the prior concomitant administration of another drug / 10. Wchich of the statements below does not properly describe incompatibility? - types of incompatibility include physical, chemical, and pharmaceutical incompatibilities./ Begum et al Asian Journal of Pharmaceutical Research and Development. 2018; 6(6): 56-61 Asian Journal of Pharmaceutical Research and Development © 2013-18, publisher and licensee AJPRD, This is an Open Access article which permits unrestricted non-commercial use, provided the original work is properly cited A J P R D Open Review Article (An International Peer-Reviewed Journal of Pharmaceutical Research and Development) Access Available online at http://ajprd.com/index.php PHARMACEUTICAL INCOMPATIBILITES: A REVIEW S.Gousia Begum*, Y.Dastagiri Reddy, B.Sri Divya, S.Jyothi Kiranmai, P.Komali, K. Sushmitha and S.Ruksar Santhiram College of Pharmacy, Affiliated to Jawaharlal Nehru Technological University, Anantapur-, Nandyal, Kurnool Dist. A.P.-518501 ABSTRACT Incompatibility is defined as a change resulting and an undesirable product is formed, which may affect the safety, efficacy, appearance and stability of the pharmaceutical product. It is of three types. It includes physical, chemical and therapeutic incompatibilities. The below described article gives the detailed information about the types, causes and how to overcome these types of incompatibilities. The occurrence of chemical incompatibilities can be overcome by two methods which include method A&B. Key words: Incompatibility,pharmaceutical product,chemical incompatibilities Article Info: Received: 23 Oct 2018; Review Completed: 12 Dec 2018; Accepted: 20 Dec 2018; Available online: 30 Dec 2018 Cite this article as: S. Gousia Begum*, Y.Dastagiri Reddy, B.SriDivya,S.JyothiKiranmai,P.Komali,K.Sushmitha and S.Ruksar, Pharmaceutical Incompatibilites: A Review, Asian Journal of Pharmaceutical research and Development.2018;6 (6): 56-61 DOI: http://dx.doi.org/10.22270/ajprd.v6i6.448 *Address for Correspondence S. Gousia Begum, Santhiram College of Pharmacy, Affiliated to Jawaharlal Nehru Technological University, Anantapur-, Nandyal, Kurnool Dist. A.P INDRODUCTION 1. Physical incompatibilities I 2. Chemical incompatibilities ncompatibility is definedas a change resulting and an undesirable product is formed, which may affect the safety, efficacy appearance and stability of the pharmaceutical product1. Incompatibilities occur during 2 3. Therapeutic incompatibilities PHYSICAL INCOMPATIBILITIES:When two or more than two substances are combined together, a physical change takes place and an unacceptable product is formed. Compounding Formulation Manufacturing Packaging Dispensing Storage Administration of drugs Interaction between two or more substances which may lead to change in color, odor, taste, viscosity and morphology. It is also called as pharmaceutical incompatibility5. Manifestations of physical incompatibility:- The incompatibilities may be detected by changes in the physical, chemical, and therapeutic qualities of the medicine. The following list outlines the various ways incompatibility between or among drug agents may be manifested. TYPES OF INCOMPATIBILITIES:- A. Insolubility:-insolubility of prescribed agents in vehicle B. Immiscibility:-Immiscibility of two or more liquids C. Precipitation:-It occurs due to solvent is insoluble when it is added to solution The incompatibilities occur when the components of a medicine interact in such a way that properties of that medicine are adversely affected3, 4. ISSN: 2320-4850 [56] CODEN (USA): AJPRHS Begum et al Asian Journal of Pharmaceutical Research and Development. 2018; 6(6): 56-61 D. Liquefaction:-Liquefaction of solids mixed in a dry state (called eutexia) INSOLUBILITY It means the inability of material to dissolve in a particular solvent system. The majority of incompatibilities is due to insolubility of the inorganic as well as organic compounds in particular solvents6. The following factors affect the solubility of prescribed agent in vehicle and may render it less soluble. Change in PH Milling Surfactant Chemical reaction Complex formation Co-solvent Any change in previous factors may lead to precipitation of drugs and change in their properties. Substances like chalk, acetyl salicylic acid, succinylsulphothiazzole, zinc oxide, and calamine are the commonexamples of in diffusible solids. Some tinctures containing resins or chlorophyll may provide precipitation when added to the aqueous system7. E.g.:-Mixture of prepared chalk E.g.:- Gelatin Arabic gum Others produce enzymes which oxidize the surfactant. Temperature Oils and water are immiscible with each other which shows physical incompatibility E.g.:- Castor oil emulsion Rx Castor oil – 15ml Water – 60ml Causes: -In this prescription castor oil is immiscible with water due to high interfacial tensions, which is a sign of incompatibility. Remedy:-To overcome this type of incompatibility emulsification is necessary with the help of an emulsifying agent. The corrected prescription is Castor oil emulsion Rx Castor oil – 15ml Acacia – 2% W/V Water– upto 60ml Rx Chalk powder –2g Tincture catechu – 2ml Cinnamon water – 2ml LIQUIFACTION Causes: - Chalk powder is not soluble in water.It gets precipitated when added to aqueous medium.These precipitates are found in diffusible in nature which results in physical incompatibility. Remedy: - Use of suspending agents is necessary to suspend the precipitated chalk particles. Generally 2% W/V of compound tragacanth powder is recommended as suspending agent. The corrected prescription is When certain low melting point solids are mixed together, a liquid or soft mass know as eutectic mixture is produced.This occurs due to the lowering of the melting point of the mixture to below room temperature and liberation of hydrates12. If such conditions take place, compounding such powders becomes difficult since the ultimate mixture turns to liquid. The medicaments showing this type of behavior are camphor, menthol, phenol, thymol, chloral hydrate, aspirin, sodium salicylates, etc…… E.g.:-Insufflations Mixture of prepared chalk Rx Rx Menthol – 5g Camphor – 5g Water – 60ml Chalk powder –2g Tragacanth – 0.4g Tincture catechu – 2ml Cinnamon water up to 30ml Causes: - This mixture is a physical incompatibility because both the ingredients in the prescription are liquefiable of mixed together. IMMISCIBILITY When two suchingredients are combined resulting in a non- homogenous product, such ingredients are called immiscible to each other and the phenomenon is called immiscibility.This manifestation appears clearly in emulsions, creams, lotions, some types of ointments. Separation in two phases is noticed in this pharmaceutical dosage form.Storage must be in room temperature to prevent separation9, 10. The following factors lead to immiscibility11 False time of addition Unsuitable for the type of emulsion Presence of micro – organisms Some bacteria grow on constituents of mixture. Hence the corrected prescription is Rx Menthol – 5g Camphor – 5g Light kaolin– 0.2g Incomplete mixing Addition of surfactant with Unsuitable concentration ISSN: 2320-4850 Remedy:-These substances can be dispensed by any one of the following method.Triturate together to form liquid and mixed with an absorbent (light kaolin, magnesium carbonate) to produce the following powder.The individual medicaments is powdered separately and mixed with an adsorbent and then combined together tightly and filled in a suitable container12. [57] CODEN (USA): AJPRHS Begum et al Asian Journal of Pharmaceutical Research and Development. 2018; 6(6): 56-61 PRECIPITATION Solubilized substances may precipitate from it solution if a non-solvent for the substances is added to the solution. E.g.:- Resins are insoluble in water Alcoholic solution of resins + water =precipitated resins. Aqueous dispersions of hydrophilic colloids (polysaccharide mucilage + high concentration of alcohol or salts) =precipitated colloids. a) High concentration of electrolytes causes cracking of soap emulsion by salting out the emulsifying agents. Vehicles (one or more organic liquids) use to dissolve medicaments of low solubility; water soluble adjuvant practically inorganic salts may be precipitated in such vehicles. Whentinctures containing resinous matter are added in water, resin agglomerates forms in diffusible precipitates. This can be prevented by slowly adding the undiluted tincture with vigorous shake.Suspension or by adding some suitable thickening agent13,14. E.g.:- Lotion of compound tincture of benzoin Rx Tincture benzoin compound – 5g Glycerin – 10ml Rose water upto 100ml Causes: - Tincture benzoin compound contain resins.This change in solvent system results in an unavoidable precipitate. Remedy: - Addition of tincture with rapid stirring yields a fine colloidal dispersion. So there is no need of any suspending agents. CHEMICAL INCOMPATIBILITIES Reaction between two or more substances which lead to change in chemical properties of pharmaceutical dosage form. As aresult of this a toxic or inactive or product may be formed15. Occurrence:- Based on natureof chemical reaction Immediate incompatibilities: - If the chemical reaction takes place, immediately after combining the prescription ingredients, they are called immediate incompatibilities. Hence, they should be dispensed only after correction. Delayed incompatibility: - When the chemical reaction proceeds at a very slow rate and no appreciable visible change occurs which may develop on keeping the product for along time are called delayed incompatibility18. Based on the prescriber Intentional:- When the prescriber knowingly prescribes the incompatible drugs. Unidirectional:- When the prescriber prescribes the drugs without knowing that there is incompatibility between the prescribed drugs19. Generally reaction between strong solution proceed at a faster rate and the precipitates are formed are thick and do not diffuse readily.Reaction between the dilute solutions proceeds at a slow rate and the precipitates formed are light and diffuse readily in the solution.Hence the reacting substances should be diluted as much as possible before mixing20. Precipitate yielding interactions The precipitates so formed may be diffusible or indiffusible. The method A or B is followed in dispensing the prescription yielding diffusible and indiffusible precipitates respectively.The preparation should contain a thickening agent if the precipitate is non-diffusible21. Method A: This method is suitable for diffusible precipitates following steps are carried out22. Divide the vehicle into two portions. Chemical incompatibilities occur, due to the chemical properties of drugs and additive like16, E.g.: substitution of caffeine citrate with caffeine in sodium salicylate and caffeine citrate mixture. PH change Oxidation-reduction reactions Acid-base hydrolysis Double decomposition Complex formation Dissolve the reactants in separate portions and mix the two portions by slowly by adding one into other with constant stirring. Method B: This method is suitable for in diffusible precipitates following steps are carried out23. These reactions may be noticed by Divide the vehicle into two portions. Dissolve the one of the reacting substance in one portion. Precipitation Effervescence Decomposition Color change Explosion Place second portion of vehicle in mortar and incorporate suitable amount of compound.Tragacanth powder (2g/100ml of preparation) with constant trituration until a smooth mucilage is produced. Add and dissolve the other reacting substance to the mucilage. TYPES OF CHEMICAL INCOMPATIBILITIES Based on chemical interactions Tolerated incompatibility: - In this type incompatibility, the chemical interactions can be changing the order of mixing the solutions indilute forms, without or by changing the order of mixing. Adjusted incompatibilities: - In adjusted incompatibility change in the formulation is needed with a compound having equal therapeutic value17. ISSN: 2320-4850 [58] Add the solution of first reactant to the mucilage slowly with constant stirring. A secondary label ―SHAKE THE BOTTLE BEFORE USE‖ should be fixed on the container whenever method A or method B is followed in dispensing the prescription. Examples of chemical incompatibilities and their correction24 CODEN (USA): AJPRHS Begum et al Asian Journal of Pharmaceutical Research and Development. 2018; 6(6): 56-61 Alkaloid incompatibility:- the action produced is different room that intended by the prescriber25. 1. Alkaloidal salts with alkaloid substances 2. Alkaloidal salts with soluble iodides 3. Alkaloidal salts with tannins 4. Alkaloid salts with salicylates 5. Alkaloid with soluble iodides and bromides. MECHANISM: It is divided into two groups. They are Pharmacokinetic: It involves the effect of a drug on another from the point of view that includes absorption, distribution, metabolism and excretion. Pharmacodynamics: These are related to the pharmacological activity of the inter-acing drugs. E.g., Synergism, antagonism, altered cellular transport, effect on the receptor site. Soluble salicylates incompatibility:1.Soluble salicylates with ferric salts 2.Soluble salicylates with alkali bicarbonates 3.Soluble salicylates and benzoates with acids. Soluble iodides incompatibility:1.Oxidation of iodides with potassium chlorate 2.Oxidation of iodides with quinine sulphate. Therapeutic incompatibilities occurs due to following reasons Chemical incompatibility causing evolution of carbon dioxide gas:1.Sodium bicarbonate with soluble calcium or magnesium salts 2.Bismuthsubnitrate and sodium bicarbonate 3.Borax with sodium bicarbonate and glycerin. Miscellaneous incompatibilities:1.Soluble barbiturates with ammonium bromide 2.Potassium chlorate with oxdisible substances 3. Incompatibility of emulsifying agent 4. Color stability of dyes 5. Incompatibilities of liquorices liquid extract a. b. c. d. e. Error in dosage Wrong dose or dosage form Contra-indicated drugs Synergistic and antagonistic drugs Drug interactions ERROR IN DOSAGE Many therapeutic incompatibilities result from errors in writing or interpreting the prescription order. The most serious type of the dosage error in the dispensing is overdose of a medication26. E.g., Atropine sulphate capsules Rx Atropine sulphate - 0.005g Phenobarbitone - 0.015g Aspirin - 0.300g Eg-1: strychnine hydrochloride mixture Rx Strychnine hydrochloride solution -6ml Aromatic spirit of ammonia -4ml Water up to - 120ml Causes:- In this prescription, the quantity of the atropine sulphate in each capsule is more than its recommended dose. Remedy:- The prescription is referred back to the prescriber to correct the overdose of the atropine sulphate. The recommended dose of atropine for a single capsule is 0.25 to 2mg. Causes: The quantity of strychnine hydrochloride is more than its solubility in water (1:30). The aromatic spirit of ammonia contains negligible amount alcohol. Remedy: - Strychnine hydrochloride gets precipitated yielding diffusible precipitate, hence follow method A. WRONFG DOSE OR DOSAGE FORM There are certain drugs which have quite similar names and there is always a danger of dispensing the wrong drug27. E.g-2.:Quinine hydrochloride mixture E.g., Prednisone and Prednisolone Rx Quinine hydrochloride Sodium salicylate Water Digoxin and Dig toxin -0.12ml -4g -100ml Some times many drugs are available in the different dosage forms and hence, if the dosage form is not clearly mentioned on the prescription, it becomes necessary to seek clarification from the prescriber. Causes: - When quinine hydrochloride combined with the sodium salicylates it forms quinine salicylates which is an in diffusible precipitate. Remedy: - Hence follow method B for precipitate yielding interactions. THERAPEUTIC INCOMPATIBILITY It is the modification of the therapeutic effect of one drug by the prior concomitant administration of another. It may be as a result of prescribing certain drugs to a patient with the intention to produce a specific degree of pharmacological action, but have restore or intensity of ISSN: 2320-4850 [59] The responsibility of the pharmacist becomes to check the prescription intensively and if he finds these types of errors he should immediately consult the prescriber for the clarification. PRESCRIBING CONTRA-INDICATED DRUGS There are certain drugs which may be contra-indicated in a particular disease or a particular patient who is allergic to it28. Corticosteroids are contra-indicated in the patients having peptic ulcers. CODEN (USA): AJPRHS Begum et al Asian Journal of Pharmaceutical Research and Development. 2018; 6(6): 56-61 The penicillin and sulphur drugs are contra-indicated in the patients who are allergic. Vasoconstrictors are contra-indicated in hypertensive patients. Barbiturates and morphine should not be given to the asthmatic patients. Causes:-In this prescription, there is a combination of two sympathomimetic drugs E.g., Sulphadiazine capsules DRUG INTERACTIONS The effect of one drug is altered by the prior or simultaneous administration of another drug. The drug interaction can usually be corrected by the proper adjustment of dosage if the suspected interaction is detected30. Causes:-Ammonium chloride is a urinary acidifier. It causes the deposition of the Sulphonamide crystals in the kidney. Remedy: - Before prescribing such substances a doctor must be careful. If he does not, a Pharmacist shows his caliber to point out such type of the doctor’s error. Such must Immediately be referred back to the concerned doctor and get corrected. PRESCRIBING SYNERGISTIC OR ANTAGONISTIC DRUGS When two drugs are prescribed together, they tend to increase the activity of each other which is known as SYNERGISM. When two drugs are prescribed together, they tend to decrease the activity of each other which is known as ANTAGONISM29. E.g., A combination of aspirin and paracetamol increases the analgesic activity. A combination of penicillin and streptomycin increases the antibacterial activity. Amphetamines show its antagonists effect with the barbiturates. There by causing additive effect. Remedy:- The prescription is referred back to the prescriber for necessary corrections. E.g., Tetracycline capsule - 250mg capsules Direction: Take one capsule every 6 hours with milk. Causes:-Tetracycline is inactivated by calcium present in milk. So, it should not be taken with milk. Remedy: In this prescription, the therapeutic incompatibility is unintentional. So, the prescription is referred back to the prescriber to change the direction. CONCLUSION: Incompatibility is defined as a change resulting and an undesirable product is formed, which may affect the safety, efficacy, appearance and stability of the pharmaceutical product. It is of three types. It includes physical, chemical and therapeutic incompatibilities. The below described article gives the detailed information about the types, causes and how to overcome these types of incompatibilities. The occurrence of chemical incompatibilities can be overcome by two methods which include method A&B. E.g., Amphetamine sulphate syrup REFERENCES: 1. Faria CE, Fiumara K, Patel N et al. Visual compatibility of furosemide with phenylephrine and vasopressin. Am J HealthSyst Pharm. 2006; 63:906-8. Letter 2. Allen LV, Levison RS, Phisutsinthop D. Compatibility of various admixtures with secondary additives at Y-injection sites of intravenous administration sets. Am J Hosp Pharm. 1977; 34:93943 3. Roche VF. Improving pharmacy students’ understanding and longterm retention of acid–base chemistry. Am J Pharm Educ. 2007; 71: Article 122. 4. Arny HV, Fischelis RP. Principles of pharmacy. 4th ed. Philadelphia, PA: W. B. Saunders; 1937:25. 5. Skau K. Pharmacy is a science-based profession. Am J Pharm Educ. 2006; 71: Article 11. 6. Newton DW. Science-based pharmacy 6. North GT, Anderson WD. Interpreting, rather than reciting, the literature on drug compatibilities. Am J Hosp Pharm. 1995; 52:1400,1404. 7. Miller KW. Coordinating and optimizing educational efforts between basic science and clinical faculty: pharmaceutics. Am J Pharm Educ. 1975; 39:576-8. 8. Burkiewicz JS. Incompatibility of ceftriaxone sodium with lactated Ringer’s injection. Am J Health-Syst Pharm. 1999; 56:384. Letter. 9. Murray L. Physicians’ desk reference. 61st ed. Montvale, NJ: Thomson PDR; 2007:1026,1030. 10. Mendenhall A, Hoyt DB. Incompatibility of ketorolac tromethamine with haloperidol lactate and thiethylperazine maleate. Am J Hosp Pharm. 1994; 51:2964. 11. Newton DW. Physicochemical determinants of incompatibility and instability in injectable drug solutions and admixtures. IsJ Hosp Pharm. 1978; 35:1213-22. 12. Newton DW. Introduction: physicochemical determinants of incompatibility and instability of drugs for injection and infusion. In: Trissel LA. Handbook on inject able drugs. 3rd ed. Bethesda, MD: American Society of Hospital Pharmacists; 1983 ISSN: 2320-4850 [60] 13. Newton DW, Narducci WA. Extemporaneous formulations. In: King RE, ed. dispensing of medication. 9th ed. Easton, PA: Mack; 1984:281-5 14. Turco SJ. Intravenous admixtures. In: Troy DB, ed. Remington: the science and practice of pharmacy. 21st ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:847. 15. O’Donnell PB, Bokser AD. Stability of pharmaceutical products. In: Troy DB, ed. Remington: the science and practice of pharmacy. 21st ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:102536. 16. Driscoll DF, Joy J, Silvestre AP et al. Calcium (Ca) and phosphate (P) compatibility in low osmolality parenteral nutrition (PN) mixtures. ClinNutr. 2005; 24:695. 17. Trissel LA. Handbook on inject able drugs. 14th ed. Bethesda, MD: American Society of Health-System Pharmacists; 2007 18. Lyman RA, Urdang G. In: Lyman RA, ed. Pharmaceutical compounding and dispensing. Philadelphia, PA: J. B. Lippincott; 1949:v. 19. Henderson LJ. A diagrammatic representation of equilibria between acids and bases in solution. J Am Chem. Soc. 1908; 30:954-60. 20. Hasselbalch KA. The calculation of the hydrogen number of the blood from the free and bound carbon dioxide of the same and the binding of oxygen by the blood as a function of the hydrogen number. Biochem Z. 1916; 78:112-44. 21. Lemke TL, Williams DA, Roche VF et al. Foye’s principles of medicinal chemistry. 6th ed. Baltimore, MD: Lippincott Williams& Wilkins; 2008:28-38,85,175. Primer Drug incompatibility357Am J Health-Syst Pharm—Vol66 Feb 15, 2009 22. Martin AN. Physical pharmacy. 4th ed. Philadelphia, PA: Lea &Febiger; 1993:223,130-2,144-6,149,169-70,179,214-5,2325,3967. 23. Newton DW, Kluza RB. Prediction of phenytoin solubility in intravenous admixtures: physicochemical theory. Is J Hosp Pharm. 1980; 37:1647-51. 24. Newton DW, Kluza RB. PKa values of medicinal compounds in pharmacy practice. Drug IntelClin Pharm. 1978; 12:546-54. CODEN (USA): AJPRHS Begum et al Asian Journal of Pharmaceutical Research and Development. 2018; 6(6): 56-61 25. Allen LV Jr, Popovich NG, Ansel HC. Ansel’s pharmaceutical dosage forms and delivery systems. 8th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2005:112, 145-6,152,338-41. 27. 26. 28. Lemke TL. Review of organic functional groups. 4th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2003:4,46-7,123, 132-40. 27. Gennaro AR. Organic pharmaceutical chemistry. In: Troy DB, ed. Remington: the science and practice of pharmacy. 21st ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:391-400. ISSN: 2320-4850 [61] 28. Lien EJ. Molecular structure, properties, and states of matter. In: Troy DB, ed. Remington: the science and practice of pharmacy. 21st ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005: 172-3. 29. Gupta PK. Solutions and phase equilibria. In: Troy DB, ed. Remington: the science and practice of pharmacy. 21st ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:2145,220-4. 30. Berge SM, Bighley LD, Monk house DL. Pharmaceutical salts. J Pharm Sci. 1977; 66:1-19. 33. CODEN (USA): AJPRHS DEFINITION Drug interaction is defined as the pharmacological activity of one drug is altered by the concominant use of another drug or by the presence of some other substance. •The Drug whose Activity is effected by such an Interaction is called as a “Object drug.” • The agent which precipitates such an interaction is refered to as the “Precipitant”. Types of drug Interactions 1.Drug-drug interactions. 2.Drug-food interactions. 3.Chemical-drug interactions. 4.Drug-laboratory test interactions. 5.Drug-disease interactions. The Net effect of a Drug Interaction is: •Generally quantitative i.e.increased or decreased effect. •Seldom qualitative i.e.rapid or slower effect. •Precipitation of newer or increased adverse effect. Drug interactions are thus• Mostly undesirable • Rarely desirable(beneficial): for eg.,enhancement of activity of penicillins when administered with probenecid. Factors contributing to drug interactions: 1.Multiple drug therapy. 2.Multiple prescribers. 3.Multiple pharmacological effects of drug. 4.Multiple diseases/predisposing illness. 5.Poor patient compliance. 6.Advancing age of patient. 7.Drug-related factors. Mechanisms of drug interactions: The three mechanisms by which an interaction can develop are1.Pharmaceutical interactions. 2.pharmacokinetic interactions. 3.Pharmacodynamic interactions. Pharmaceutical interactions: Also called as incompatibility.it is a physicochemical interaction that occous when drugs are mixed in i.v . Infusions causing precipitation or inactivation of active principles . Example:Ampicillin ,chlorpromazine &barbituates interact with dextran in solutions and are broken down or from chemical compounds. Pharmacokinetic Interactions: “These interactions are those in which adme properties of the object drug is altered by the precipitant and hence such interactions are also called as ADME interactions”. •The resultant effect is altered plasma concentration of the object drug. •These are classified as: 1.Absorption interactions 2.Distribution interactions 3.Metabolism interactions 4.Excretion interactions. Absorption interactions Are those where the absorption . of the object drug is altered. The net effect of such an interaction is: • Faster or slower drug absorption. • More, or, less complete drug absorption. Major mechanisms of absorption interactions are: 1.Complexation and adsorption. 2.Alteration in GI pH. 3.Alteration in gut motility. 4.Inhibition of GI enzymes. 5.Alteration of GI micro flora. 6.Malabsorption syndrome. OBJECT DRUG PRECIPITANT DRUGS INFLUENCE ON OBJECT DRUG ABSORPTION INTERACTION 1.COMPLEXATION & ADSORPTION CEPHROFLOXACINE, PENCILLAMINE ANTACIDS,FOOD & MINERALS SUPPLEMENTS CONTAINING AL,Mg,Fe,Zn & Ca IONS FORMATION OF POORELY SOLUBLE AND UNABSOBABLE COMPLEX WITH SUCH HEAVY METAL IONS. 2.ALTERATION OF GI PH SULPHONAMIDES, ASPIRIN FERROUS SULPHATE ANTACIDS ENHANCED DISSOLUTION AND ABSORPTION RATE. SODIUM BICARBONATE,CALCIUM CARBONATE DECREASED DISOLLUTION AND HENCE ABSORPTION. 3.ALTERATION OF GUT MOTILITY ASPIRIN DIAZEPAM, LEVODOPA, MEXILETINE LEVODOPA, LITHIUM CARBONATE, MEXILETINE METOCLOPRAMIDE RAPID GASTRIC EMPTYING,INCREASED RATE OF ABSORPION. ANTI CHOLINERGICS DELAYED GASTRIC EMPTYING;DECREASED RATE OF ABSORPTION. OBJECT DRUG PRECIPITANT DRUGS INFLUENCE ON OBJECT DRUG 4.ALTERATION OF GI MICROFLORA DIGOXIN ANTI BIOTICS INCREASED BIOAVAILABILITY DUE TO DESTRUCTION OF BACTERIAL FLORA THAT INACTIVATES DIGOXIN IN LOWER INTESTINE. 5.MALABSORPTION SNDROME VITAMIN A,B12,DIGOXIN NEOMYCIN INHIBITION OF ABSORPTION DUE TO MAL. Distribution interactions Are those where the distribution pattern of the object drug is altered : •The major mechanism for distribution interaction is alteration in protein-drug binding. Competitive displacement interactions Displaced drug Displacer Anti coagulants Tolbutamide Phenylbutazone, chloral hydrate Sulphonamides Increased clotting tme. increased risk of hemorrhage. Increased hypoglycemic effect. METABOLISM INTERACTIONS: Are those where the metabolism of the object drug is altered. Mechanisms of metabolism interactions include: 1.Enzyme induction: Increased rate of metabolism. 2.Enzyme inhibition: Decreased rate of metabolism. It is the most significant interaction in comparison to other interactions and can be fatal. METABOLISM INTERACTIONS 1.ENZYNE INDUCTION CORTICOSTEROIDS, ORAL CONTRACEPTIVES, COUMARINS, PHENYTOIN ORAL CONTRACEPTIVES, ORAL HYPOGLYCAEMICS BARBITURATES RIFAMICIN DECREASED PLASMA LEVELS; DECREASED EFFICASY OF OBJECT DRUGS DECREASED PLASMA LEVELS 2.ENZYME INHIBITION TYRAMINE RICH FOOD MAO INHIBITORS ENHANCED ABSORPTION OF UN METABOLISED TYRAMINE. COUMARINS METRANIDAZOLE PHENYL BUTAZONE INCREASED ANTI COAGULANT ACTIVITY. ALCOHOL DISULPHIRAM, METRONIDAZOLE INCREASED IN PLASMA ACETALDEHYDE LEVELS EXCRETION INTERACTIONS Are these where the excretion pattern of the object drug is altered. Major mechanisms of excretion interactions areAlteration in renal blood flow Alteration of urine PH Competition for active secretions Forced diuresis EXCRETION INTERACTIONS 1.CHANGES IN ACTIVE TUBULAR SECRETION PENCILLIN,CEPHALOSP ORINS,NALIDIXIC ACID PROBENICID ELEVATED PLASMA LEVELS OF ACIDIC DRUGS 2.CHANGES IN URINE PH AMPHETAMINE ANTACIDS,THIAZIDESA CETAZOLAMIDE INCREASED PASSIVE REABSORPTION OF BASIC DRUGS.INCRESED RISK Of TOXICITY 3.CHANGES IN RENAL BLOOD FLOW LITHIUM BICARBONATE NSAIDS DECREASED RENAL CLEARANCEOF LITHIUM.RISK OF TOXICITY Pharmacodynamic interactions: Are those in which the activity of the object drug at its site of action is altered by the precipitant. Such interactions may be direct or indirect. 1.These are of two types1.direct pharmacodynamic interactions. 2.Indirect pharmacodynamic interactions. DIRECT PHARMACODYNAMIC INTERACTIONS: In which drugs having similar or opposing pharmacological effects are used concurrently. The three consequences of direct interactions are 1.Antagonism. 2.Addition or summation. 3.Synergism or potentiation. Antagonism: The interacting drugs have opposing actions Example: Acetylcholine and noradrenaline have opposing effects on heart rate. Addition or summation: The interacting drugs have similar actions and the resultant effect is the some of individual drug responses Example:CNS depressants like sedatives and hypnotics,…etc Synergism or potentiation: It is an enhancement of action of one drug by another Example: Alcohol enhances the analgesics activity of aspirin. Indirect pharmacodynamic interaction: In which both the object and the precipitant drugs have unrelated effects.but the latter in Some way alerts the effects but latter in some way alerts the effectsof the former. Example: salicylatesdecrease the ability of the platelets to aggregate thus impairing the Homeostasis if warfarin indused bleeding occurs. CONSEQUENCES OF DRUG INTERACTIONS: The consequences of drug interactions may be: •Major: Life threatening. •Moderate: Deteriotion of patients status. •Minor: Little effect. REDUSING THE RISK OF DRUG INTERACTIONS: 1.Identify the patients risk factors. 2.Take through drug history. 3.Be knowledge about the actions of the drugs being used. 4.Consider therapeutic alternatives. 5Avoid complex therapeutic regiments when possible. 6.Educate the patient. 7.Monitor therapy. INFLUENCE OF SMOKING ON DRUG INTERACTIONS: Smoking increases the activity of drug metabolizing enzymes in the liver, With the result that certain therapeutic agents. Example: Diazepam, propoxyphene, theophylline, olanzapine. Are metabolized more rapidly,and their effect is decreased. INFLUENCE OF ALCOHOL ON DRUG INTERACTION: Chronic use of alcohol beverages may increases the rate of metabolism of drugs such as warfarin and phenytoin, probably by increasing the activity of hepatic enzymes. •Acute use of alcohol by non alcoholic individuals may cause an inhibition of hepatic enzymes. •Use of alcoholic beverages with sedatives and other depressants drugs could result in an excessive depressant response. INFLUENCE OF FOOD ON DRUG INTERACTION: Food effects the rate and extent of absorption of drugs from the GI tract. Example: Many anti biotics should be given atleast 1hr before or 2hr after meals to achieve Optimal absorption. •The type of food may be important with regard to the absorption of concurrently administered Drugs. Example: Dietary items such as milk and other dairy products that contain calcium may decrease . The absorption of tetracycline and flouroquinolone derivatives. •Diet also may influence urinary pH values.