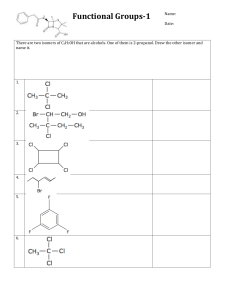
Q1. What is the IUPAC name for this compound? A 2-dimethyl-3-fluoropentane B 2,2-dimethyl-3-fluoropentane C 3-fluoro-2,2-dimethylpentane D 3-fluoro-2-dimethylpentane (Total 1 mark) Q2. The correct systematic name for is A 2-ethyl-3,4-dimethylpent-2-ene B 4-ethyl-2,3-dimethylpent-3-ene C 2,3,4-trirnethylhex-3-ene D 3,4,5-trimethylhex-3-ene (Total 1 mark) Q3. Which can be both an empirical and molecular formula of a stable compound? A CH2O B P4O10 C NH2 D CH3 (Total 1 mark) Q4. The correct name for the alkene monomer which forms the polymer shown below is Page 1 of 7 A 2-methyl-3-ethylpropene B 2-methylpent-2-ene C 2-methylpent-3-ene D 4-methylpent-2-ene (Total 1 mark) Q5. The correct systematic name for is A 2,3-diethylbut-2-ene B 2-ethyl-3-methylpent-2-ene C 4-ethyl-3-methylpent-3-ene D 3,4-dimethylhex-3-ene (Total 1 mark) Q6. CH2O is the empirical formula of A methanol B methyl methanoate C ethane-1,2-diol D butanal (Total 1 mark) Q7. Which compound has the lowest relative molecular mass? A ethanoic acid B 1-fluoropropane C propanenitrile Page 2 of 7 D propylamine (Total 1 mark) Q8. Which one of the following is the correct name for A 2-bromo-3-methylpent-2-ene B 2-bromo-3-ethylbut-2-ene C 3-bromo-2-ethylbut-2-ene D 4-bromo-3-methylpent-3-ene ? (Total 1 mark) Q9. The structural formula of ethyl 2-methylpropanoate is A B C D (Total 1 mark) Q10. This question is about citric acid, a hydrated tricarboxylic acid. Its formula can be represented as H3Y.xH2O (a) A 1.50 g sample of H3Y.xH2O contains 0.913 g of oxygen by mass. The sample burns completely in air to form 1.89 g of CO2 and 0.643 g of H2O Show that the empirical formula of citric acid is C3H5O4 Page 3 of 7 (5) (b) A 3.00 g sample of H3Y.xH2O (Mr = 210.0) is heated to constant mass. The anhydrous H3Y that remains has a mass of 2.74 g Show, using these data, that the value of x = 1 (2) The figure shows the structure of H3Y (c) Complete this IUPAC name for H3Y ____________________ propane-1, 2, 3-tricarboxylic acid (1) (d) State the number of peaks you would expect in the 13C NMR spectrum for H3Y ___________________________________________________________________ (1) (Total 9 marks) Q11. Isomers X and Y have the molecular formula C5H8O Give the IUPAC name for isomer X. ___________________________________________________________________ (1) Page 4 of 7 (Total 1 mark) Q12. This question is about the structures of some organic molecules. (a) Draw the skeletal formula of 3-methylbutanal. (1) (b) Draw the displayed formula of C5H11Br that is the major product of the reaction of 2methylbut-2-ene with hydrogen bromide. (1) (c) Thermal cracking of hydrocarbons produces molecules that are attacked by electrophiles because they have a region of high electron density. Draw the structure of one of these molecules that contains four carbon atoms. (1) (Total 3 marks) Q13. The table below gives some of the names and structures of isomers having the molecular formula C4H9Br Structure Name Page 5 of 7 CH3CH2CH2CH2Br 2-bromo - 2-methypropane 1-bromo - 2-methypropane 2-methypropane Complete the table. (Total 2 marks) Q14. Four isomers with the formula C4H9OH are given below. Isomer Name CH3CH2CH2CH2OH butan-1-ol 2-methylpropan-2-ol (i) Complete the naming of the isomers in the table above. Page 6 of 7 (ii) Name the type of isomerism shown by these four isomers. ___________________________________________________________________ (Total 3 marks) Page 7 of 7